Methods to detect infectious human enteric viruses in environmental water samples
- PMID: 21920815
- PMCID: PMC7106513
- DOI: 10.1016/j.ijheh.2011.07.014
Methods to detect infectious human enteric viruses in environmental water samples
Abstract
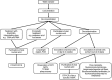
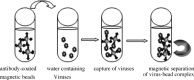
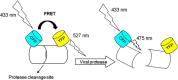
Currently, a wide range of analytical methods is available for virus detection in environmental water samples. Molecular methods such as polymerase chain reaction (PCR) and quantitative real time PCR (qPCR) have the highest sensitivity and specificity to investigate virus contamination in water, so they are the most commonly used in environmental virology. Despite great sensitivity of PCR, the main limitation is the lack of the correlation between the detected viral genome and viral infectivity, which limits conclusions regarding the significance for public health. To provide information about the infectivity of the detected viruses, cultivation on animal cell culture is the gold standard. However, cell culture infectivity assays are laborious, time consuming and costly. Also, not all viruses are able to produce cytopathic effect and viruses such as human noroviruses have no available cell line for propagation. In this brief review, we present a summary and critical evaluation of different approaches that have been recently proposed to overcome limitations of the traditional cell culture assay and PCR assay such as integrated cell culture-PCR, detection of genome integrity, detection of capsid integrity, and measurement of oxidative damages on viral capsid protein. Techniques for rapid detection of infectious viruses such as fluorescence microscopy and automated flow cytometry have also been suggested to assess virus infectivity in water samples.
Copyright © 2011 Elsevier GmbH. All rights reserved.
Figures

Similar articles
-
Critical issues in application of molecular methods to environmental virology.J Virol Methods. 2019 Apr;266:11-24. doi: 10.1016/j.jviromet.2019.01.008. Epub 2019 Jan 16. J Virol Methods. 2019. PMID: 30659861 Review.
-
Development of a quantitative immunocapture real-time PCR assay for detecting structurally intact adenoviral particles in water.J Virol Methods. 2013 Dec;194(1-2):235-41. doi: 10.1016/j.jviromet.2013.07.009. Epub 2013 Jul 11. J Virol Methods. 2013. PMID: 23850702
-
Integrated cell culture/PCR for detection of enteric viruses in environmental samples.Methods Mol Biol. 2004;268:69-78. doi: 10.1385/1-59259-766-1:069. Methods Mol Biol. 2004. PMID: 15156019
-
Environmental virology: from detection of virus in sewage and water by isolation to identification by molecular biology--a trip of over 50 years.Annu Rev Microbiol. 1995;49:461-87. doi: 10.1146/annurev.mi.49.100195.002333. Annu Rev Microbiol. 1995. PMID: 8561468 Review.
-
Viral persistence in surface and drinking water: Suitability of PCR pre-treatment with intercalating dyes.Water Res. 2016 Mar 15;91:68-76. doi: 10.1016/j.watres.2015.12.049. Epub 2015 Dec 31. Water Res. 2016. PMID: 26773484
Cited by
-
Quantification of human and animal viruses to differentiate the origin of the fecal contamination present in environmental samples.Biomed Res Int. 2013;2013:192089. doi: 10.1155/2013/192089. Epub 2013 May 15. Biomed Res Int. 2013. PMID: 23762826 Free PMC article.
-
Common and Potential Emerging Foodborne Viruses: A Comprehensive Review.Life (Basel). 2024 Jan 28;14(2):190. doi: 10.3390/life14020190. Life (Basel). 2024. PMID: 38398699 Free PMC article. Review.
-
Methods to prevent PCR amplification of DNA from non-viable virus were not successful for infectious laryngotracheitis virus.PLoS One. 2020 May 22;15(5):e0232571. doi: 10.1371/journal.pone.0232571. eCollection 2020. PLoS One. 2020. PMID: 32442180 Free PMC article.
-
Coronaviruses and SARS-CoV-2 in sewerage and their removal: Step by step in wastewater treatment plants.Environ Res. 2022 May 1;207:112204. doi: 10.1016/j.envres.2021.112204. Epub 2021 Oct 14. Environ Res. 2022. PMID: 34656637 Free PMC article. Review.
-
Propidium Monoazide Integrated with qPCR Enables the Detection and Enumeration of Infectious Enteric RNA and DNA Viruses in Clam and Fermented Sausages.Front Microbiol. 2016 Dec 15;7:2008. doi: 10.3389/fmicb.2016.02008. eCollection 2016. Front Microbiol. 2016. PMID: 28018329 Free PMC article.
References
-
- Abd El Galil K.H., El Sokkary M.A., Kheira S.M., Salazar A.M., Yates M.V., Chen W., Mulchandani A. Combined immunomagnetic separation-molecular beacon-reverse transcription-PCR assay for detection of hepatitis A virus from environmental samples. Appl. Environ. Microbiol. 2004;70:4371–4374. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

