Protein-intrinsic and signaling network-based sources of resistance to EGFR- and ErbB family-targeted therapies in head and neck cancer
- PMID: 21920801
- PMCID: PMC3195944
- DOI: 10.1016/j.drup.2011.08.002
Protein-intrinsic and signaling network-based sources of resistance to EGFR- and ErbB family-targeted therapies in head and neck cancer
Abstract
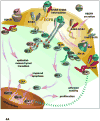
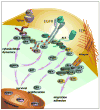
Agents targeting EGFR and related ErbB family proteins are valuable therapies for the treatment of many cancers. For some tumor types, including squamous cell carcinomas of the head and neck (SCCHN), antibodies targeting EGFR were the first protein-directed agents to show clinical benefit, and remain a standard component of clinical strategies for management of the disease. Nevertheless, many patients display either intrinsic or acquired resistance to these drugs; hence, major research goals are to better understand the underlying causes of resistance, and to develop new therapeutic strategies that boost the impact of EGFR/ErbB inhibitors. In this review, we first summarize current standard use of EGFR inhibitors in the context of SCCHN, and described new agents targeting EGFR currently moving through pre-clinical and clinical development. We then discuss how changes in other transmembrane receptors, including IGF1R, c-Met, and TGF-β, can confer resistance to EGFR-targeted inhibitors, and discuss new agents targeting these proteins. Moving downstream, we discuss critical EGFR-dependent effectors, including PLC-γ; PI3K and PTEN; SHC, GRB2, and RAS and the STAT proteins, as factors in resistance to EGFR-directed inhibitors and as alternative targets of therapeutic inhibition. We summarize alternative sources of resistance among cellular changes that target EGFR itself, through regulation of ligand availability, post-translational modification of EGFR, availability of EGFR partners for hetero-dimerization and control of EGFR intracellular trafficking for recycling versus degradation. Finally, we discuss new strategies to identify effective therapeutic combinations involving EGFR-targeted inhibitors, in the context of new system level data becoming available for analysis of individual tumors.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
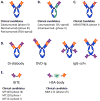


Similar articles
-
Targeting pathways mediating resistance to anti-EGFR therapy in squamous cell carcinoma of the head and neck.Expert Rev Anticancer Ther. 2016 Aug;16(8):847-58. doi: 10.1080/14737140.2016.1202116. Epub 2016 Jul 11. Expert Rev Anticancer Ther. 2016. PMID: 27400139 Review.
-
New approaches to EGFR inhibition for locally advanced or metastatic squamous cell carcinoma of the head and neck (SCCHN).Med Oncol. 2012 Dec;29(4):2481-91. doi: 10.1007/s12032-012-0159-2. Epub 2012 Jan 18. Med Oncol. 2012. PMID: 22252310 Free PMC article. Review.
-
Recent insights in the PI3K/Akt pathway as a promising therapeutic target in combination with EGFR-targeting agents to treat head and neck squamous cell carcinoma.Med Res Rev. 2022 Jan;42(1):112-155. doi: 10.1002/med.21806. Epub 2021 Apr 29. Med Res Rev. 2022. PMID: 33928670 Review.
-
Combined targeting of epidermal growth factor receptor, signal transducer and activator of transcription-3, and Bcl-X(L) enhances antitumor effects in squamous cell carcinoma of the head and neck.Mol Pharmacol. 2008 Jun;73(6):1632-42. doi: 10.1124/mol.107.044636. Epub 2008 Mar 6. Mol Pharmacol. 2008. PMID: 18326051 Free PMC article.
-
Molecular phenotype predicts sensitivity of squamous cell carcinoma of the head and neck to epidermal growth factor receptor inhibition.Mol Oncol. 2013 Jun;7(3):359-68. doi: 10.1016/j.molonc.2012.11.001. Epub 2012 Nov 14. Mol Oncol. 2013. PMID: 23200321 Free PMC article.
Cited by
-
Progress in Precision Medicine for Head and Neck Cancer.Cancers (Basel). 2024 Nov 4;16(21):3716. doi: 10.3390/cancers16213716. Cancers (Basel). 2024. PMID: 39518152 Free PMC article. Review.
-
Epidermal growth factor (EGF) regulates α5β1 integrin activation state in human cancer cell lines through the p90RSK-dependent phosphorylation of filamin A.J Biol Chem. 2012 Nov 23;287(48):40371-80. doi: 10.1074/jbc.M112.389577. Epub 2012 Sep 24. J Biol Chem. 2012. PMID: 23007402 Free PMC article.
-
Activation of MET pathway predicts poor outcome to cetuximab in patients with recurrent or metastatic head and neck cancer.J Transl Med. 2015 Aug 29;13:282. doi: 10.1186/s12967-015-0633-7. J Transl Med. 2015. PMID: 26319934 Free PMC article.
-
IFIT1 and IFIT3 promote oral squamous cell carcinoma metastasis and contribute to the anti-tumor effect of gefitinib via enhancing p-EGFR recycling.Oncogene. 2019 Apr;38(17):3232-3247. doi: 10.1038/s41388-018-0662-9. Epub 2019 Jan 9. Oncogene. 2019. PMID: 30626937
-
Tumor Biomarkers in Spindle Cell Variant Squamous Cell Carcinoma of the Head and Neck.Otolaryngol Head Neck Surg. 2016 Jul;155(1):106-12. doi: 10.1177/0194599816636612. Epub 2016 Mar 15. Otolaryngol Head Neck Surg. 2016. PMID: 26980915 Free PMC article.
References
-
- Abidoye OO, Cohen EE, Wong SJ, et al. Phase II study of lapatinib (GW572016) in recurrent/metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) J Clin Oncol (ASCO Ann Meet Proc, Part 1) 2006;24 (18S):5568.
-
- Adams GP, Schier R, McCall, et al. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 2001;61:4750–4755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA06927/CA/NCI NIH HHS/United States
- R01 GM84453/GM/NIGMS NIH HHS/United States
- R01 CA063366/CA/NCI NIH HHS/United States
- R01 CA113342/CA/NCI NIH HHS/United States
- R01 GM084453-09/GM/NIGMS NIH HHS/United States
- R01 GM084453/GM/NIGMS NIH HHS/United States
- R01-CA63366/CA/NCI NIH HHS/United States
- R01 CA063366-17/CA/NCI NIH HHS/United States
- R01-CA113342/CA/NCI NIH HHS/United States
- P30 CA006927/CA/NCI NIH HHS/United States
- R01 CA113342-05/CA/NCI NIH HHS/United States
- P30 CA006927-48/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

