Cfr and RlmN contain a single [4Fe-4S] cluster, which directs two distinct reactivities for S-adenosylmethionine: methyl transfer by SN2 displacement and radical generation
- PMID: 21916495
- PMCID: PMC3596424
- DOI: 10.1021/ja207327v
Cfr and RlmN contain a single [4Fe-4S] cluster, which directs two distinct reactivities for S-adenosylmethionine: methyl transfer by SN2 displacement and radical generation
Abstract
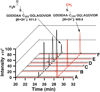
The radical SAM (RS) proteins RlmN and Cfr catalyze methylation of carbons 2 and 8, respectively, of adenosine 2503 in 23S rRNA. Both reactions are similar in scope, entailing the synthesis of a methyl group partially derived from S-adenosylmethionine (SAM) onto electrophilic sp(2)-hybridized carbon atoms via the intermediacy of a protein S-methylcysteinyl (mCys) residue. Both proteins contain five conserved Cys residues, each required for turnover. Three cysteines lie in a canonical RS CxxxCxxC motif and coordinate a [4Fe-4S]-cluster cofactor; the remaining two are at opposite ends of the polypeptide. Here we show that each protein contains only the one "radical SAM" [4Fe-4S] cluster and the two remaining conserved cysteines do not coordinate additional iron-containing species. In addition, we show that, while wild-type RlmN bears the C355 mCys residue in its as-isolated state, RlmN that is either engineered to lack the [4Fe-4S] cluster by substitution of the coordinating cysteines or isolated from Escherichia coli cultured under iron-limiting conditions does not bear a C355 mCys residue. Reconstitution of the [4Fe-4S] cluster on wild-type apo RlmN followed by addition of SAM results in rapid production of S-adenosylhomocysteine (SAH) and the mCys residue, while treatment of apo RlmN with SAM affords no observable reaction. These results indicate that in Cfr and RlmN, SAM bound to the unique iron of the [4Fe-4S] cluster displays two reactivities. It serves to methylate C355 of RlmN (C338 of Cfr), or to generate the 5'-deoxyadenosyl 5'-radical, required for substrate-dependent methyl synthase activity.
© 2011 American Chemical Society
Figures

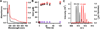
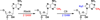
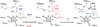
Similar articles
-
Structural basis for methyl transfer by a radical SAM enzyme.Science. 2011 May 27;332(6033):1089-92. doi: 10.1126/science.1205358. Epub 2011 Apr 28. Science. 2011. PMID: 21527678 Free PMC article.
-
RlmN and Cfr are radical SAM enzymes involved in methylation of ribosomal RNA.J Am Chem Soc. 2010 Mar 24;132(11):3953-64. doi: 10.1021/ja910850y. J Am Chem Soc. 2010. PMID: 20184321 Free PMC article.
-
Radical-mediated enzymatic methylation: a tale of two SAMS.Acc Chem Res. 2012 Apr 17;45(4):555-64. doi: 10.1021/ar200202c. Epub 2011 Nov 18. Acc Chem Res. 2012. PMID: 22097883 Free PMC article. Review.
-
A radically different mechanism for S-adenosylmethionine-dependent methyltransferases.Science. 2011 Apr 29;332(6029):604-7. doi: 10.1126/science.1200877. Epub 2011 Mar 17. Science. 2011. PMID: 21415317
-
Auxiliary iron-sulfur cofactors in radical SAM enzymes.Biochim Biophys Acta. 2015 Jun;1853(6):1316-34. doi: 10.1016/j.bbamcr.2015.01.002. Epub 2015 Jan 15. Biochim Biophys Acta. 2015. PMID: 25597998 Review.
Cited by
-
Radical SAM-mediated methylation reactions.Curr Opin Chem Biol. 2013 Aug;17(4):597-604. doi: 10.1016/j.cbpa.2013.05.032. Epub 2013 Jul 5. Curr Opin Chem Biol. 2013. PMID: 23835516 Free PMC article. Review.
-
Cysteine methylation controls radical generation in the Cfr radical AdoMet rRNA methyltransferase.PLoS One. 2013 Jul 5;8(7):e67979. doi: 10.1371/journal.pone.0067979. Print 2013. PLoS One. 2013. PMID: 23861844 Free PMC article.
-
Probing the coordination and function of Fe4S4 modules in nitrogenase assembly protein NifB.Nat Commun. 2018 Jul 19;9(1):2824. doi: 10.1038/s41467-018-05272-8. Nat Commun. 2018. PMID: 30026506 Free PMC article.
-
Radical S-adenosylmethionine enzymes.Chem Rev. 2014 Apr 23;114(8):4229-317. doi: 10.1021/cr4004709. Epub 2014 Jan 29. Chem Rev. 2014. PMID: 24476342 Free PMC article. Review. No abstract available.
-
Structural diversity in the AdoMet radical enzyme superfamily.Biochim Biophys Acta. 2012 Nov;1824(11):1178-95. doi: 10.1016/j.bbapap.2012.04.006. Epub 2012 Apr 28. Biochim Biophys Acta. 2012. PMID: 22579873 Free PMC article. Review.
References
-
- Frey PA, Hegeman AD. New York: Oxford University Press; 2007.
-
- Markham GD. Encyclopedia of Life Sciences. John Wiley & Sons, Inc; 2010. S-adenosylmethionine.
-
- Silverman RB. New York: Academic Press; 2002.
-
- Hegazi MF, Borchardt RT, Schowen RL. J. Am. Chem. Soc. 1979;101:4359–4365.
-
- Iwig DF, Grippe AT, McIntyre TA, Booker SJ. Biochemistry. 2004;43(42):13510–13524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

