Transgenic expression of human LAMA5 suppresses murine Lama5 mRNA and laminin α5 protein deposition
- PMID: 21915268
- PMCID: PMC3168496
- DOI: 10.1371/journal.pone.0023926
Transgenic expression of human LAMA5 suppresses murine Lama5 mRNA and laminin α5 protein deposition
Abstract
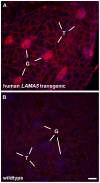
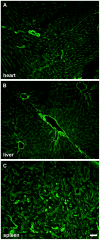
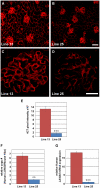
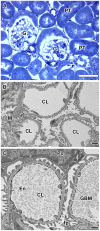
Laminin α5 is required for kidney glomerular basement membrane (GBM) assembly, and mice with targeted deletions of the Lama5 gene fail to form glomeruli. As a tool to begin to understand factors regulating the expression of the LAMA5 gene, we generated transgenic mice carrying the human LAMA5 locus in a bacterial artificial chromosome. These mice deposited human laminin α5 protein into basement membranes in heart, liver, spleen and kidney. Here, we characterized two lines of transgenics; Line 13 expressed ∼6 times more LAMA5 than Line 25. Mice from both lines were healthy, and kidney function and morphology were normal. Examination of developing glomeruli from fetal LAMA5 transgenics showed that the human transgene was expressed at the correct stage of glomerular development, and deposited into the nascent GBM simultaneously with mouse laminin α5. Expression of human LAMA5 did not affect the timing of the mouse laminin α1-α5 isoform switch, or that for mouse laminin β1-β2. Immunoelectron microscopy showed that human laminin α5 originated in both glomerular endothelial cells and podocytes, known to be origins for mouse laminin α5 normally. Notably, in neonatal transgenics expressing the highest levels of human LAMA5, there was a striking reduction of mouse laminin α5 protein in kidney basement membranes compared to wildtype, and significantly lower levels of mouse Lama5 mRNA. This suggests the presence in kidney of a laminin expression monitor, which may be important for regulating the overall production of basement membrane protein.
Conflict of interest statement
Figures
Similar articles
-
Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane.J Cell Biol. 2003 Apr 14;161(1):187-96. doi: 10.1083/jcb.200211121. Epub 2003 Apr 7. J Cell Biol. 2003. PMID: 12682087 Free PMC article.
-
Defective glomerulogenesis in the absence of laminin alpha5 demonstrates a developmental role for the kidney glomerular basement membrane.Dev Biol. 2000 Jan 15;217(2):278-89. doi: 10.1006/dbio.1999.9546. Dev Biol. 2000. PMID: 10625553
-
Laminin compensation in collagen alpha3(IV) knockout (Alport) glomeruli contributes to permeability defects.J Am Soc Nephrol. 2007 Sep;18(9):2465-72. doi: 10.1681/ASN.2007030328. Epub 2007 Aug 15. J Am Soc Nephrol. 2007. PMID: 17699809
-
Ultrastructure of developing kidney glomerular basement membranes: temporal changes in binding of anti-laminin IgG and cationized ferritin.Microsc Res Tech. 1994 Jun 1;28(2):81-94. doi: 10.1002/jemt.1070280202. Microsc Res Tech. 1994. PMID: 8054666 Review.
-
The glomerular basement membrane.Exp Cell Res. 2012 May 15;318(9):973-8. doi: 10.1016/j.yexcr.2012.02.031. Epub 2012 Mar 5. Exp Cell Res. 2012. PMID: 22410250 Free PMC article. Review.
Cited by
-
Maternal alloimmune IgG causes anti-glomerular basement membrane disease in perinatal transgenic mice that express human laminin α5.Kidney Int. 2019 Dec;96(6):1320-1331. doi: 10.1016/j.kint.2019.06.014. Epub 2019 Jul 10. Kidney Int. 2019. PMID: 31530475 Free PMC article.
-
Assessing complex movement behaviors in rodent models of neurological disorders.Neurobiol Learn Mem. 2019 Nov;165:106817. doi: 10.1016/j.nlm.2018.02.025. Epub 2018 Feb 21. Neurobiol Learn Mem. 2019. PMID: 29476821 Free PMC article.
-
Role of the podocyte (and glomerular endothelium) in building the GBM.Semin Nephrol. 2012 Jul;32(4):342-9. doi: 10.1016/j.semnephrol.2012.06.005. Semin Nephrol. 2012. PMID: 22958488 Free PMC article. Review.
-
Functional consequences of cell type-restricted expression of laminin α5 in mouse placental labyrinth and kidney glomerular capillaries.PLoS One. 2012;7(7):e41348. doi: 10.1371/journal.pone.0041348. Epub 2012 Jul 20. PLoS One. 2012. PMID: 22911783 Free PMC article.
References
-
- Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–487. - PubMed
-
- Haraldsson B, Jeansson M. Glomerular filtration barrier. Curr Opin Nephrol Hypertens. 2009;18:331–335. - PubMed
-
- Patrakka J, Tryggvason K. Molecular make-up of the glomerular filtration barrier. Biochem Biophys Res Commun. 2010;396:164–169. - PubMed
-
- Jeansson M, Haraldsson B. Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. Am J Physiol Renal Physiol. 2006;290:F111–F116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

