The myosin-binding UCS domain but not the Hsp90-binding TPR domain of the UNC-45 chaperone is essential for function in Caenorhabditis elegans
- PMID: 21914819
- PMCID: PMC3706032
- DOI: 10.1242/jcs.087320
The myosin-binding UCS domain but not the Hsp90-binding TPR domain of the UNC-45 chaperone is essential for function in Caenorhabditis elegans
Abstract
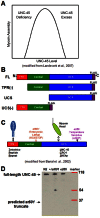
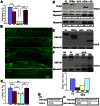
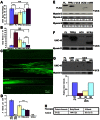
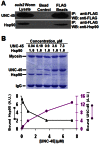
The UNC-45 family of molecular chaperones is expressed in metazoan organisms from Caenorhabditis elegans to humans. The UNC-45 protein is essential in C. elegans for early body-wall muscle cell development and A-band assembly. We show that the myosin-binding UCS domain of UNC-45 alone is sufficient to rescue lethal unc-45 null mutants arrested in embryonic muscle development and temperature-sensitive loss-of-function unc-45 mutants defective in worm A-band assembly. Removal of the Hsp90-binding TPR domain of UNC-45 does not affect rescue. Similar results were obtained with overexpression of the same fragments in wild-type nematodes when assayed for diminution of myosin accumulation and assembly. Titration experiments show that, on a per molecule basis, UCS has greater activity in C. elegans muscle in vivo than full-length UNC-45 protein, suggesting that UNC-45 is inhibited by either the TPR domain or its interaction with the general chaperone Hsp90. In vitro experiments with purified recombinant C. elegans Hsp90 and UNC-45 proteins show that they compete for binding to C. elegans myosin. Our in vivo genetic and in vitro biochemical experiments are consistent with a novel inhibitory role for Hsp90 with respect to UNC-45 action.
Figures
Similar articles
-
Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin.Science. 2002 Jan 25;295(5555):669-71. doi: 10.1126/science.1066648. Science. 2002. PMID: 11809970
-
Knockdown and overexpression of Unc-45b result in defective myofibril organization in skeletal muscles of zebrafish embryos.BMC Cell Biol. 2010 Sep 17;11:70. doi: 10.1186/1471-2121-11-70. BMC Cell Biol. 2010. PMID: 20849610 Free PMC article.
-
The myosin chaperone UNC-45 is organized in tandem modules to support myofilament formation in C. elegans.Cell. 2013 Jan 17;152(1-2):183-95. doi: 10.1016/j.cell.2012.12.025. Cell. 2013. PMID: 23332754 Free PMC article.
-
The UNC-45 myosin chaperone: from worms to flies to vertebrates.Int Rev Cell Mol Biol. 2014;313:103-44. doi: 10.1016/B978-0-12-800177-6.00004-9. Int Rev Cell Mol Biol. 2014. PMID: 25376491 Free PMC article. Review.
-
UCS proteins: chaperones for myosin and co-chaperones for Hsp90.Subcell Biochem. 2015;78:133-52. doi: 10.1007/978-3-319-11731-7_7. Subcell Biochem. 2015. PMID: 25487020 Review.
Cited by
-
UNC-45B chaperone: the role of its domains in the interaction with the myosin motor domain.Biophys J. 2014 Aug 5;107(3):654-661. doi: 10.1016/j.bpj.2014.05.045. Biophys J. 2014. PMID: 25099804 Free PMC article.
-
Myosin-dependent cell-cell communication controls synchronicity of division in acute and chronic stages of Toxoplasma gondii.Nat Commun. 2017 Jun 8;8:15710. doi: 10.1038/ncomms15710. Nat Commun. 2017. PMID: 28593938 Free PMC article.
-
A novel conserved isoform of the ubiquitin ligase UFD2a/UBE4B is expressed exclusively in mature striated muscle cells.PLoS One. 2011;6(12):e28861. doi: 10.1371/journal.pone.0028861. Epub 2011 Dec 9. PLoS One. 2011. PMID: 22174917 Free PMC article.
-
Mutations in conserved residues of the myosin chaperone UNC-45 result in both reduced stability and chaperoning activity.Protein Sci. 2021 Nov;30(11):2221-2232. doi: 10.1002/pro.4180. Epub 2021 Sep 28. Protein Sci. 2021. PMID: 34515376 Free PMC article.
-
UCS protein Rng3p is essential for myosin-II motor activity during cytokinesis in fission yeast.PLoS One. 2013 Nov 14;8(11):e79593. doi: 10.1371/journal.pone.0079593. eCollection 2013. PLoS One. 2013. PMID: 24244528 Free PMC article.
References
-
- Baker K. E., Parker R. (2004). Nonsense-mediated mRNA decay: terminating erroneous gene expression. Curr. Opin. Cell Biol. 16, 293-299 - PubMed
-
- Barral J. M., Hutagalung A. H., Brinker A., Hartl F. U., Epstein H. F. (2002). Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science 295, 669-671 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

