Allele-dependent processing pathways generate the endogenous human leukocyte antigen (HLA) class I peptide repertoire in transporters associated with antigen processing (TAP)-deficient cells
- PMID: 21914809
- PMCID: PMC3207402
- DOI: 10.1074/jbc.M111.281808
Allele-dependent processing pathways generate the endogenous human leukocyte antigen (HLA) class I peptide repertoire in transporters associated with antigen processing (TAP)-deficient cells
Abstract
The transporters associated with antigen processing (TAP) allow the supply of peptides derived from the cytosol to translocate to the endoplasmic reticulum, where they complex with nascent human leukocyte antigen (HLA) class I molecules. However, infected and tumor cells with TAP molecules blocked or individuals with nonfunctional TAP complexes are able to present HLA class I ligands generated by TAP-independent processing pathways. These peptides are detected by the CD8(+) lymphocyte cellular response. Here, the generation of the overall peptide repertoire associated with four different HLA class I molecules in TAP-deficient cells was studied. Using different protease inhibitors, four different proteolytic specificities were identified. These data demonstrate the different allele-dependent complex processing pathways involved in the generation of the HLA class I peptide repertoire in TAP-deficient cells.
Figures
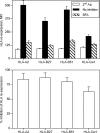
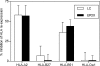
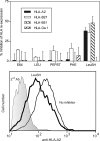
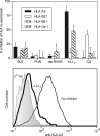
Similar articles
-
Diversity of natural self-derived ligands presented by different HLA class I molecules in transporter antigen processing-deficient cells.PLoS One. 2013;8(3):e59118. doi: 10.1371/journal.pone.0059118. Epub 2013 Mar 26. PLoS One. 2013. PMID: 23555621 Free PMC article.
-
Targeting tumour cells with defects in the MHC Class I antigen processing pathway with CD8+ T cells specific for hydrophobic TAP- and Tapasin-independent peptides: the requirement for directed access into the ER.Cancer Immunol Immunother. 2007 Aug;56(8):1143-52. doi: 10.1007/s00262-006-0263-2. Epub 2006 Dec 2. Cancer Immunol Immunother. 2007. PMID: 17143611 Free PMC article.
-
A viral, transporter associated with antigen processing (TAP)-independent, high affinity ligand with alternative interactions endogenously presented by the nonclassical human leukocyte antigen E class I molecule.J Biol Chem. 2012 Oct 12;287(42):34895-34903. doi: 10.1074/jbc.M112.362293. Epub 2012 Aug 27. J Biol Chem. 2012. PMID: 22927436 Free PMC article.
-
Endogenous TAP-independent MHC-I antigen presentation: not just the ER lumen.Curr Opin Immunol. 2020 Jun;64:9-14. doi: 10.1016/j.coi.2019.12.003. Epub 2020 Jan 11. Curr Opin Immunol. 2020. PMID: 31935516 Review.
-
Vaccination and the TAP-independent antigen processing pathways.Expert Rev Vaccines. 2013 Sep;12(9):1077-83. doi: 10.1586/14760584.2013.825447. Expert Rev Vaccines. 2013. PMID: 24053400 Review.
Cited by
-
Stability and Expression Levels of HLA-C on the Cell Membrane Modulate HIV-1 Infectivity.J Virol. 2017 Dec 14;92(1):e01711-17. doi: 10.1128/JVI.01711-17. Print 2018 Jan 1. J Virol. 2017. PMID: 29070683 Free PMC article.
-
CAR-T cells targeting a nucleophosmin neoepitope exhibit potent specific activity in mouse models of acute myeloid leukaemia.Nat Biomed Eng. 2021 May;5(5):399-413. doi: 10.1038/s41551-020-00625-5. Epub 2020 Oct 12. Nat Biomed Eng. 2021. PMID: 33046866 Free PMC article.
-
Role of metalloproteases in vaccinia virus epitope processing for transporter associated with antigen processing (TAP)-independent human leukocyte antigen (HLA)-B7 class I antigen presentation.J Biol Chem. 2012 Mar 23;287(13):9990-10000. doi: 10.1074/jbc.M111.314856. Epub 2012 Feb 1. J Biol Chem. 2012. PMID: 22298786 Free PMC article.
-
Alternative Antigen Processing for MHC Class I: Multiple Roads Lead to Rome.Front Immunol. 2015 Jun 5;6:298. doi: 10.3389/fimmu.2015.00298. eCollection 2015. Front Immunol. 2015. PMID: 26097483 Free PMC article. Review.
-
Diversity of natural self-derived ligands presented by different HLA class I molecules in transporter antigen processing-deficient cells.PLoS One. 2013;8(3):e59118. doi: 10.1371/journal.pone.0059118. Epub 2013 Mar 26. PLoS One. 2013. PMID: 23555621 Free PMC article.
References
-
- Seifert U., Marañón C., Shmueli A., Desoutter J. F., Wesoloski L., Janek K., Henklein P., Diescher S., Andrieu M., de la Salle H., Weinschenk T., Schild H., Laderach D., Galy A., Haas G., Kloetzel P. M., Reiss Y., Hosmalin A. (2003) Nat. Immunol. 4, 375–379 - PubMed
-
- Guil S., Rodríguez-Castro M., Aguilar F., Villasevil E. M., Antón L. C., Del Val M. (2006) J. Biol. Chem. 281, 39925–39934 - PubMed
-
- York I. A., Bhutani N., Zendzian S., Goldberg A. L., Rock K. L. (2006) J. Immunol. 177, 1434–1443 - PubMed
-
- Stoltze L., Schirle M., Schwarz G., Schröter C., Thompson M. W., Hersh L. B., Kalbacher H., Stevanovic S., Rammensee H. G., Schild H. (2000) Nat. Immunol. 1, 413–418 - PubMed
-
- Parmentier N., Stroobant V., Colau D., de Diesbach P., Morel S., Chapiro J., van Endert P., Van den Eynde B. J. (2010) Nat. Immunol. 11, 449–454 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

