Coordination of the Rab5 cycle on macropinosomes
- PMID: 21910808
- PMCID: PMC3213305
- DOI: 10.1111/j.1600-0854.2011.01280.x
Coordination of the Rab5 cycle on macropinosomes
Abstract
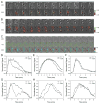
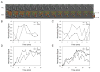
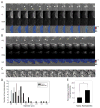
The GTPase Rab5a regulates the homotypic and heterotypic fusion of membranous organelles during the early stages of endocytosis. Many of the molecules which regulate the Rab5a cycle of association with membranes, activation, deactivation and dissociation are known. However, the extent to which these molecular scale activities are coordinated on membranes to affect the behavior of individual organelles has not been determined. This study used novel Förster resonance energy transfer (FRET) microscopic methods to analyze the Rab5a cycle on macropinosomes, which are large endocytic vesicles that form in ruffled regions of cell membranes. In Cos-7 cells and mouse macrophages stimulated with growth factors, Rab5a activation followed immediately after its recruitment to newly formed macropinosomes. Rab5a activity increased continuously and uniformly over macropinosome membranes then decreased continuously, with Rab5a deactivation preceding dissociation by 1-12 min. Although the maximal levels of Rab5a activity were independent of organelle size, Rab5a cycles were longer on larger macropinosomes, consistent with an integrative activity governing Rab5a dynamics on individual organelles. The Rab5a cycle was destabilized by microtubule depolymerization and by bafilomycin A1. Overexpression of activating and inhibitory proteins indicated that active Rab5a stabilized macropinosomes. Thus, overall Rab5a activity on macropinosomes is coordinated by macropinosome structure and physiology.
© 2011 John Wiley & Sons A/S.
Figures


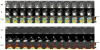
Similar articles
-
Delivery of CSF-1R to the lumen of macropinosomes promotes its destruction in macrophages.J Cell Sci. 2014 Dec 15;127(Pt 24):5228-39. doi: 10.1242/jcs.154393. Epub 2014 Oct 21. J Cell Sci. 2014. PMID: 25335894 Free PMC article.
-
Dynamics of rab5 activation in endocytosis and phagocytosis.J Leukoc Biol. 2000 Nov;68(5):627-32. J Leukoc Biol. 2000. PMID: 11073100
-
Rab5 activation on macropinosomes requires ALS2, and subsequent Rab5 inactivation through ALS2 detachment requires active Rab7.FEBS Lett. 2019 Jan;593(2):230-241. doi: 10.1002/1873-3468.13306. Epub 2018 Dec 7. FEBS Lett. 2019. PMID: 30485418
-
[Effectors of GTPase Rab5 in endocytosis and signal transduction].Postepy Biochem. 2009;55(2):171-80. Postepy Biochem. 2009. PMID: 19824473 Review. Polish.
-
Shaping tubular carriers for intracellular membrane transport.FEBS Lett. 2009 Dec 3;583(23):3847-56. doi: 10.1016/j.febslet.2009.10.031. Epub 2009 Oct 17. FEBS Lett. 2009. PMID: 19840796 Review.
Cited by
-
All Roads Lead to Cytosol: Trypanosoma cruzi Multi-Strategic Approach to Invasion.Front Cell Infect Microbiol. 2021 Mar 5;11:634793. doi: 10.3389/fcimb.2021.634793. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33747982 Free PMC article. Review.
-
Delivery of CSF-1R to the lumen of macropinosomes promotes its destruction in macrophages.J Cell Sci. 2014 Dec 15;127(Pt 24):5228-39. doi: 10.1242/jcs.154393. Epub 2014 Oct 21. J Cell Sci. 2014. PMID: 25335894 Free PMC article.
-
Quantitative contributions of processes by which polyanion drugs reduce intracellular bioavailability and transfection efficiency of cationic siRNA lipoplex.J Control Release. 2018 Jan 28;270:101-113. doi: 10.1016/j.jconrel.2017.12.001. Epub 2017 Dec 22. J Control Release. 2018. PMID: 29203416 Free PMC article.
-
Small GTPases control macropinocytosis of amyloid precursor protein and cleavage to amyloid-β.Heliyon. 2024 May 11;10(10):e31077. doi: 10.1016/j.heliyon.2024.e31077. eCollection 2024 May 30. Heliyon. 2024. PMID: 38799759 Free PMC article.
-
Small GTPases and phosphoinositides in the regulatory mechanisms of macropinosome formation and maturation.Front Physiol. 2014 Sep 30;5:374. doi: 10.3389/fphys.2014.00374. eCollection 2014. Front Physiol. 2014. PMID: 25324782 Free PMC article. Review.
References
-
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. - PubMed
-
- Perskvist N, Roberg K, Kulyte A, Stendahl O. Rab5a GTPase regulates fusion between pathogen-containing phagosomes and cytoplasmic organelles in human neutrophils. J Cell Sci. 2002;115:1321–1330. - PubMed
-
- Bucci C, Lutcke A, Steele-Mortimer O, Olkkonen VM, Dupree P, Chiariello M, Bruni CB, Simons K, Zerial M. Co-operative regulation of endocytosis by three Rab5 isoforms. FEBS Lett. 1995;366:65–71. - PubMed
-
- Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. - PubMed
-
- Mu FT, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo JP, Tock EP, Toh BH. EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J Biol Chem. 1995;270:13503–13511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

