Upregulation of cellular Bcl-2 by the KSHV encoded RTA promotes virion production
- PMID: 21901143
- PMCID: PMC3162012
- DOI: 10.1371/journal.pone.0023892
Upregulation of cellular Bcl-2 by the KSHV encoded RTA promotes virion production
Abstract
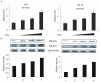
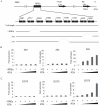
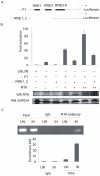
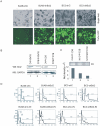
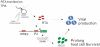
Apoptosis of virus infected cells can restrict or dampen full blown virus propagation and this can serve as a protective mechanism against virus infection. Consequently, viruses can also delay programmed cell death by enhancing the expression of anti-apoptotic proteins. Human Bcl-2 is expressed on the surface of the mitochondrial membrane and functions as the regulator of the delicate balance between cell survival and apoptosis. In this report, we showed that the replication and transcription activator (RTA) encoded by KSHV ORF 50, a key regulator for KSHV reactivation from latent to lytic infection, upregulates the mRNA and protein levels of Bcl-2 in 293 cells, and TPA-induced KSHV-infected cells. Further analysis revealed that upregulation of the cellular Bcl-2 promoter by RTA is dose-dependent and acts through targeting of the CCN(9)GG motifs within the Bcl-2 promoter. The Bcl-2 P2 but not the P1 promoter is primarily responsive to RTA. The results of ChIP confirmed the direct interaction of RTA protein with the CCN(9)GG motifs. Knockdown of cellular Bcl-2 by lentivirus-delivered small hairpin RNA (shRNA) resulted in increased cell apoptosis and decreased virion production in KSHV-infected cells. These findings provide an insight into another mechanism by which KSHV utilizes the intrinsic apoptosis signaling pathways for prolonging the survival of lytically infected host cells to allow for maximum production of virus progeny.
Conflict of interest statement
Figures
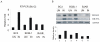
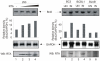
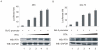
Similar articles
-
Genome-Wide Identification of Direct RTA Targets Reveals Key Host Factors for Kaposi's Sarcoma-Associated Herpesvirus Lytic Reactivation.J Virol. 2019 Feb 19;93(5):e01978-18. doi: 10.1128/JVI.01978-18. Print 2019 Mar 1. J Virol. 2019. PMID: 30541837 Free PMC article.
-
Suppressive regulation of KSHV RTA with O-GlcNAcylation.J Biomed Sci. 2012 Feb 2;19(1):12. doi: 10.1186/1423-0127-19-12. J Biomed Sci. 2012. PMID: 22300411 Free PMC article.
-
CCAAT/enhancer-binding protein-alpha is induced during the early stages of Kaposi's sarcoma-associated herpesvirus (KSHV) lytic cycle reactivation and together with the KSHV replication and transcription activator (RTA) cooperatively stimulates the viral RTA, MTA, and PAN promoters.J Virol. 2003 Sep;77(17):9590-612. doi: 10.1128/jvi.77.17.9590-9612.2003. J Virol. 2003. PMID: 12915572 Free PMC article.
-
An alternative Kaposi's sarcoma-associated herpesvirus replication program triggered by host cell apoptosis.J Virol. 2012 Apr;86(8):4404-19. doi: 10.1128/JVI.06617-11. Epub 2012 Feb 15. J Virol. 2012. PMID: 22345480 Free PMC article.
-
The Rta/Orf50 transactivator proteins of the gamma-herpesviridae.Curr Top Microbiol Immunol. 2007;312:71-100. doi: 10.1007/978-3-540-34344-8_3. Curr Top Microbiol Immunol. 2007. PMID: 17089794 Review.
Cited by
-
The Modulation of Apoptotic Pathways by Gammaherpesviruses.Front Microbiol. 2016 Apr 27;7:585. doi: 10.3389/fmicb.2016.00585. eCollection 2016. Front Microbiol. 2016. PMID: 27199919 Free PMC article. Review.
-
Kaposi sarcoma-associated herpesvirus g protein-coupled receptor enhances endothelial cell survival in part by upregulation of bcl-2.Ochsner J. 2013 Spring;13(1):66-75. Ochsner J. 2013. PMID: 23532945 Free PMC article.
-
Epstein-Barr virus essential antigen EBNA3C attenuates H2AX expression.J Virol. 2014 Apr;88(7):3776-88. doi: 10.1128/JVI.03568-13. Epub 2014 Jan 15. J Virol. 2014. PMID: 24429368 Free PMC article.
-
Silencing of semaphorin 3C suppresses cell proliferation and migration in MCF-7 breast cancer cells.Oncol Lett. 2017 Nov;14(5):5913-5917. doi: 10.3892/ol.2017.6920. Epub 2017 Sep 8. Oncol Lett. 2017. PMID: 29113226 Free PMC article.
-
Molecular biology of KSHV lytic reactivation.Viruses. 2015 Jan 14;7(1):116-53. doi: 10.3390/v7010116. Viruses. 2015. PMID: 25594835 Free PMC article. Review.
References
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Sicence. 1994;266:1865–1869. - PubMed
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. - PubMed
-
- Boshoff C, Schulz TF, Kennedy MM, Graham AK, Fisher C, et al. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274–1278. - PubMed
-
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. - PubMed
-
- Ohtsuki Y, Iwata J, Furihata M, Takeuchi T, Sonobe H, et al. Ultrastructure of Kaposi's sarcoma-associated herpesvirus (KSHV)/human herpesvirus-8 (HHV-8) in a primary effusion lymphoma cell line treated with tetradecanoyl phorbol acetate (TPA). Med Electron Microsc. 1999;32:94–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1R01CA138434-01A209/CA/NCI NIH HHS/United States
- 5R01CA091792-08/CA/NCI NIH HHS/United States
- R01 CA138434/CA/NCI NIH HHS/United States
- 1R01CA137894-01/CA/NCI NIH HHS/United States
- R01 CA091792/CA/NCI NIH HHS/United States
- R01 AI067037/AI/NIAID NIH HHS/United States
- 5R01CA108461-05/CA/NCI NIH HHS/United States
- 5R01AI067037-04/AI/NIAID NIH HHS/United States
- R01 DE017338/DE/NIDCR NIH HHS/United States
- 5R01DE017338-03/DE/NIDCR NIH HHS/United States
- R01 CA108461/CA/NCI NIH HHS/United States
- R01 CA137894/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources

