Ubiquitination-deubiquitination balance dictates ligand-stimulated PTHR sorting
- PMID: 21898592
- PMCID: PMC3222777
- DOI: 10.1002/jbmr.494
Ubiquitination-deubiquitination balance dictates ligand-stimulated PTHR sorting
Abstract
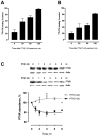
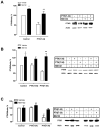
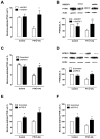
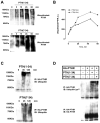
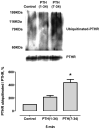
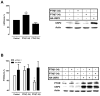
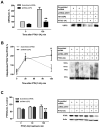
Parathyroid hormone receptors (PTHR) are promptly internalized upon stimulation by activating (PTH[1-84], PTH[1-34]) and non-activating (PTH[7-84], PTH[7-34]) ligands. Here, we characterized the mechanism regulating the sorting of internalized receptors between recycling and degradative pathways. PTHR recycles faster after challenge with PTH(1-34) than with PTH(7-34). PTHR recycling is complete by 2 h after PTH(1-34) stimulation, but incomplete at this time in cells treated with PTH(7-34). The slower and incomplete recycling induced by PTH(7-34) is due to proteasomal degradation. Both PTH(1-34) and PTH(7-34) induced PTHR polyubiquitination. Ubiquitination by PTH(1-34) was transient, whereas receptor ubiquitination after PTH(7-34) was sustained. PTH(1-34), but not PTH(7-34), induced expression of the PTHR-specific deubiquitinating enzyme USP2. Overexpression of USP2 prevented PTH(7-34)-induced PTHR degradation. We conclude that PTH(1-34) promotes coupled PTHR ubiquitination and deubiquitination, whereas PTH(7-34) activates only ubiquitination, thereby leading to PTHR downregulation. These findings may explain PTH resistance in diseases associated with elevated PTH(7-84) levels.
Copyright © 2011 American Society for Bone and Mineral Research.
Conflict of interest statement
Conflict of interests: No authors have conflicts of interest
Figures
Similar articles
-
Quantitative analysis of agonist-dependent parathyroid hormone receptor trafficking in whole cells using a functional green fluorescent protein conjugate.J Cell Physiol. 2001 Dec;189(3):341-55. doi: 10.1002/jcp.10028. J Cell Physiol. 2001. PMID: 11748592
-
Parathyroid hormone receptor recycling: role of receptor dephosphorylation and beta-arrestin.Mol Endocrinol. 2002 Dec;16(12):2720-32. doi: 10.1210/me.2002-0049. Mol Endocrinol. 2002. PMID: 12456793
-
Dual regulation of the parathyroid hormone (PTH)/PTH-related peptide receptor signaling by protein kinase C and beta-arrestins.Endocrinology. 2002 Oct;143(10):3854-65. doi: 10.1210/en.2002-220232. Endocrinology. 2002. PMID: 12239097
-
Regulation of endocytic sorting by ESCRT-DUB-mediated deubiquitination.Cell Biochem Biophys. 2011 Jun;60(1-2):39-46. doi: 10.1007/s12013-011-9181-9. Cell Biochem Biophys. 2011. PMID: 21448666 Review.
-
Deubiquitinating enzymes are IN/(trinsic to proteasome function).Curr Protein Pept Sci. 2004 Jun;5(3):201-11. doi: 10.2174/1389203043379756. Curr Protein Pept Sci. 2004. PMID: 15188770 Review.
Cited by
-
Ubiquitination of G protein-coupled receptors: functional implications and drug discovery.Mol Pharmacol. 2012 Oct;82(4):563-70. doi: 10.1124/mol.112.079418. Epub 2012 Jun 14. Mol Pharmacol. 2012. PMID: 22700696 Free PMC article. Review.
-
USP2-Related Cellular Signaling and Consequent Pathophysiological Outcomes.Int J Mol Sci. 2021 Jan 26;22(3):1209. doi: 10.3390/ijms22031209. Int J Mol Sci. 2021. PMID: 33530560 Free PMC article. Review.
-
Minireview: ubiquitination-regulated G protein-coupled receptor signaling and trafficking.Mol Endocrinol. 2013 Apr;27(4):558-72. doi: 10.1210/me.2012-1404. Epub 2013 Mar 7. Mol Endocrinol. 2013. PMID: 23471539 Free PMC article. Review.
-
Ubiquitin-specific peptidases: Players in bone metabolism.Cell Prolif. 2023 Aug;56(8):e13444. doi: 10.1111/cpr.13444. Epub 2023 Mar 8. Cell Prolif. 2023. PMID: 36883930 Free PMC article. Review.
-
Regulation of G Protein-Coupled Receptors by Ubiquitination.Int J Mol Sci. 2017 Apr 27;18(5):923. doi: 10.3390/ijms18050923. Int J Mol Sci. 2017. PMID: 28448471 Free PMC article. Review.
References
-
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. - PubMed
-
- Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:462–470. - PubMed
-
- Malecz N, Bambino T, Bencsik M, Nissenson RA. Identification of phosphorylation sites in the G protein-coupled receptor for parathyroid hormone. Receptor phosphorylation is not required for agonist-induced internalization. Mol Endocrinol. 1998;12:1846–1856. - PubMed
-
- Chauvin S, Bencsik M, Bambino T, Nissenson RA. PTH receptor recycling: role of receptor dephosphorylation and β-arrestin. Mol Endocrinol. 2002;16:2720–2732. - PubMed
-
- Tawfeek HA, Qian F, Abou-Samra AB. Phosphorylation of the receptor for PTH and PTHrP is required for internalization and regulates receptor signaling. Mol Endocrinol. 2002;16:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

