A wheat homolog of MOTHER OF FT AND TFL1 acts in the regulation of germination
- PMID: 21896881
- PMCID: PMC3203438
- DOI: 10.1105/tpc.111.088492
A wheat homolog of MOTHER OF FT AND TFL1 acts in the regulation of germination
Abstract
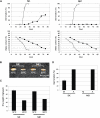
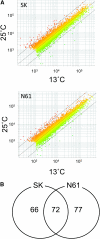
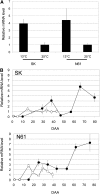
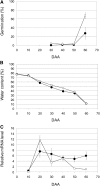
Seed dormancy is an adaptive mechanism and an important agronomic trait. Temperature during seed development strongly affects seed dormancy in wheat (Triticum aestivum) with lower temperatures producing higher levels of seed dormancy. To identify genes important for seed dormancy, we used a wheat microarray to analyze gene expression in embryos from mature seeds grown at lower and higher temperatures. We found that a wheat homolog of MOTHER OF FT AND TFL1 (MFT) was upregulated after physiological maturity in dormant seeds grown at the lower temperature. In situ hybridization analysis indicated that MFT was exclusively expressed in the scutellum and coleorhiza. Mapping analysis showed that MFT on chromosome 3A (MFT-3A) colocalized with the seed dormancy quantitative trait locus (QTL) QPhs.ocs-3A.1. MFT-3A expression levels in a dormant cultivar used for the detection of the QTL were higher after physiological maturity; this increased expression correlated with a single nucleotide polymorphism in the promoter region. In a complementation analysis, high levels of MFT expression were correlated with a low germination index in T1 seeds. Furthermore, precocious germination of isolated immature embryos was suppressed by transient introduction of MFT driven by the maize (Zea mays) ubiquitin promoter. Taken together, these results suggest that MFT plays an important role in the regulation of germination in wheat.
Figures



Similar articles
-
Seed maturation regulators are related to the control of seed dormancy in wheat (Triticum aestivum L.).PLoS One. 2014 Sep 11;9(9):e107618. doi: 10.1371/journal.pone.0107618. eCollection 2014. PLoS One. 2014. PMID: 25211528 Free PMC article.
-
A Causal Gene for Seed Dormancy on Wheat Chromosome 4A Encodes a MAP Kinase Kinase.Curr Biol. 2016 Mar 21;26(6):782-7. doi: 10.1016/j.cub.2016.01.063. Epub 2016 Mar 3. Curr Biol. 2016. PMID: 26948878
-
Functional Analysis of Seed Dormancy Genes by Biolistic Transient Gene Expression in Immature Embryos of Wheat.Methods Mol Biol. 2024;2830:131-136. doi: 10.1007/978-1-0716-3965-8_12. Methods Mol Biol. 2024. PMID: 38977574
-
Wheat grain preharvest sprouting and late maturity alpha-amylase.Planta. 2014 Dec;240(6):1167-78. doi: 10.1007/s00425-014-2172-5. Epub 2014 Sep 26. Planta. 2014. PMID: 25257145 Review.
-
Functional genomics of seed dormancy in wheat: advances and prospects.Front Plant Sci. 2014 Sep 15;5:458. doi: 10.3389/fpls.2014.00458. eCollection 2014. Front Plant Sci. 2014. PMID: 25309557 Free PMC article. Review.
Cited by
-
MKK3 Cascade Regulates Seed Dormancy Through a Negative Feedback Loop Modulating ABA Signal in Rice.Rice (N Y). 2024 Jan 3;17(1):2. doi: 10.1186/s12284-023-00679-4. Rice (N Y). 2024. PMID: 38170405 Free PMC article.
-
Expression of 9-cis-EPOXYCAROTENOID DIOXYGENASE4 is essential for thermoinhibition of lettuce seed germination but not for seed development or stress tolerance.Plant Cell. 2013 Mar;25(3):884-900. doi: 10.1105/tpc.112.108902. Epub 2013 Mar 15. Plant Cell. 2013. PMID: 23503626 Free PMC article.
-
The wheat Phs-A1 pre-harvest sprouting resistance locus delays the rate of seed dormancy loss and maps 0.3 cM distal to the PM19 genes in UK germplasm.J Exp Bot. 2016 Jul;67(14):4169-78. doi: 10.1093/jxb/erw194. Epub 2016 May 23. J Exp Bot. 2016. PMID: 27217549 Free PMC article.
-
Trend, population structure, and trait mapping from 15 years of national varietal trials of UK winter wheat.G3 (Bethesda). 2022 Feb 4;12(2):jkab415. doi: 10.1093/g3journal/jkab415. G3 (Bethesda). 2022. PMID: 34897454 Free PMC article.
-
Genome-wide association study and quantitative trait loci mapping of seed dormancy in common wheat (Triticum aestivum L.).Planta. 2019 Jul;250(1):187-198. doi: 10.1007/s00425-019-03164-9. Epub 2019 Apr 10. Planta. 2019. PMID: 30972483
References
-
- Abe M., Kobayashi Y., Yamamoto S., Daimon Y., Yamaguchi A., Ikeda Y., Ichinoki H., Notaguchi M., Goto K., Araki T. (2005). FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056 - PubMed
-
- Allouis S., Moore G., Bellec A., Sharp R., Faivre-Rampant P., Mortimer K., Pateyron S., Foote T.N., Griffiths S., Caboche M., Chaloub B. (2003). Construction and characterization of a hexaploid wheat BAC library from the reference germplasm ‘Chinese Spring’. Cereal Res. Commun. 31: 331–338
-
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. (1990). Basic local alignment search tool. J. Mol. Biol. 215: 403–410 - PubMed
-
- Appleford N.E.J., Lenton J.R. (1997). Hormonal regulation of -amylase gene expression in germinating wheat (Triticum aestivum) grains. Physiol. Plant. 100: 534–542
-
- Ashikawa I., Abe F., Nakamura S. (2010). Ectopic expression of wheat and barley DOG1-like genes promotes seed dormancy in Arabidopsis. Plant Sci. 179: 536–542 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

