The vitamin K-dependent carboxylase generates γ-carboxylated glutamates by using CO2 to facilitate glutamate deprotonation in a concerted mechanism that drives catalysis
- PMID: 21896484
- PMCID: PMC3248010
- DOI: 10.1074/jbc.M111.249177
The vitamin K-dependent carboxylase generates γ-carboxylated glutamates by using CO2 to facilitate glutamate deprotonation in a concerted mechanism that drives catalysis
Abstract
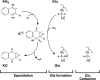
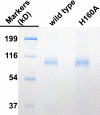
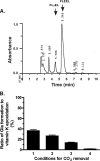
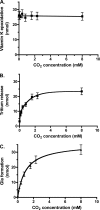
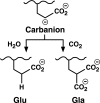
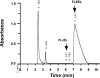
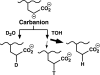
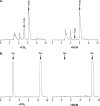
The γ-glutamyl carboxylase converts Glu to carboxylated Glu (Gla) to activate a large number of vitamin K-dependent proteins with diverse functions, and this broad physiological impact makes it critical to understand the mechanism of carboxylation. Gla formation is thought to occur in two independent steps (i.e. Glu deprotonation to form a carbanion that then reacts with CO(2)), based on previous studies showing unresponsiveness of Glu deprotonation to CO(2). However, our recent studies on the kinetic properties of a variant enzyme (H160A) showing impaired Glu deprotonation prompted a reevaluation of this model. Glu deprotonation monitored by tritium release from the glutamyl γ-carbon was dependent upon CO(2), and a proportional increase in both tritium release and Gla formation occurred over a range of CO(2) concentrations. This discrepancy with the earlier studies using microsomes is probably due to the known accessibility of microsomal carboxylase to water, which reprotonates the carbanion. In contrast, tritium incorporation experiments with purified carboxylase showed very little carbanion reprotonation and consequently revealed the dependence of Glu deprotonation on CO(2). Cyanide stimulated Glu deprotonation and carbanion reprotonation to the same extent in wild type enzyme but not in the H160A variant. Glu deprotonation that depends upon CO(2) but that also occurs when water or cyanide are present strongly suggests a concerted mechanism facilitated by His-160 in which an electrophile accepts the negative charge on the developing carbanion. This revised mechanism provides important insight into how the carboxylase catalyzes the reaction by avoiding the formation of a high energy discrete carbanion.
Figures
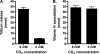
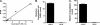
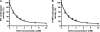
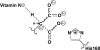
Similar articles
-
Insight into the coupling mechanism of the vitamin K-dependent carboxylase: mutation of histidine 160 disrupts glutamic acid carbanion formation and efficient coupling of vitamin K epoxidation to glutamic acid carboxylation.Biochemistry. 2008 Sep 16;47(37):9836-46. doi: 10.1021/bi800296r. Epub 2008 Aug 22. Biochemistry. 2008. PMID: 18717596 Free PMC article.
-
The propeptide of the vitamin K-dependent carboxylase substrate accelerates formation of the gamma-glutamyl carbanion intermediate.Biochemistry. 1997 May 27;36(21):6384-90. doi: 10.1021/bi962816b. Biochemistry. 1997. PMID: 9174354
-
Vitamin K-dependent carboxylase. Demonstration of a vitamin K- and O2-dependent exchange of 3H from 3H2O into glutamic acid residues.J Biol Chem. 1983 Oct 25;258(20):12129-31. J Biol Chem. 1983. PMID: 6138349
-
The vitamin K-dependent carboxylase.Annu Rev Nutr. 2005;25:127-49. doi: 10.1146/annurev.nutr.25.050304.092713. Annu Rev Nutr. 2005. PMID: 16011462 Review.
-
Structure and mechanism of action of the vitamin K-dependent gamma-glutamyl carboxylase: recent advances from mutagenesis studies.Thromb Haemost. 1997 Jul;78(1):595-8. Thromb Haemost. 1997. PMID: 9198222 Review.
Cited by
-
The vitamin K oxidoreductase is a multimer that efficiently reduces vitamin K epoxide to hydroquinone to allow vitamin K-dependent protein carboxylation.J Biol Chem. 2013 Nov 1;288(44):31556-66. doi: 10.1074/jbc.M113.497297. Epub 2013 Aug 5. J Biol Chem. 2013. PMID: 23918929 Free PMC article.
-
Vitamin K oxygenation, glutamate carboxylation, and processivity: defining the three critical facets of catalysis by the vitamin K-dependent carboxylase.Adv Nutr. 2012 Mar 1;3(2):135-48. doi: 10.3945/an.111.001719. Adv Nutr. 2012. PMID: 22516721 Free PMC article. Review.
-
Interaction of 17β-estradiol and dietary fatty acids on energy and glucose homeostasis in female mice.Nutr Neurosci. 2018 Dec;21(10):715-728. doi: 10.1080/1028415X.2017.1347374. Epub 2017 Jul 7. Nutr Neurosci. 2018. PMID: 28686546 Free PMC article.
-
A conformational investigation of propeptide binding to the integral membrane protein γ-glutamyl carboxylase using nanodisc hydrogen exchange mass spectrometry.Biochemistry. 2014 Mar 11;53(9):1511-20. doi: 10.1021/bi401536m. Epub 2014 Feb 26. Biochemistry. 2014. PMID: 24512177 Free PMC article.
References
-
- Berkner K. L. (2005) Annu. Rev. Nutr. 25, 127–149 - PubMed
-
- Morris D. P., Stevens R. D., Wright D. J., Stafford D. W. (1995) J. Biol. Chem. 270, 30491–30498 - PubMed
-
- Stenina O., Pudota B. N., McNally B. A., Hommema E. L., Berkner K. L. (2001) Biochemistry 40, 10301–10309 - PubMed
-
- Brenner B., Sánchez-Vega B., Wu S. M., Lanir N., Stafford D. W., Solera J. (1998) Blood 92, 4554–4559 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

