The surfactant of Legionella pneumophila Is secreted in a TolC-dependent manner and is antagonistic toward other Legionella species
- PMID: 21890700
- PMCID: PMC3194911
- DOI: 10.1128/JB.05405-11
The surfactant of Legionella pneumophila Is secreted in a TolC-dependent manner and is antagonistic toward other Legionella species
Abstract
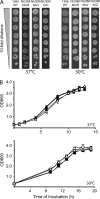
When Legionella pneumophila grows on agar plates, it secretes a surfactant that promotes flagellum- and pilus-independent "sliding" motility. We isolated three mutants that were defective for surfactant. The first two had mutations in genes predicted to encode cytoplasmic enzymes involved in lipid metabolism. These genes mapped to two adjacent operons that we designated bbcABCDEF and bbcGHIJK. Backcrossing and complementation confirmed the importance of the bbc genes and suggested that the Legionella surfactant is lipid containing. The third mutant had an insertion in tolC. TolC is the outer membrane part of various trimolecular complexes involved in multidrug efflux and type I protein secretion. Complementation of the tolC mutant restored sliding motility. Mutants defective for an inner membrane partner of TolC also lacked a surfactant, confirming that TolC promotes surfactant secretion. L. pneumophila (lspF) mutants lacking type II protein secretion (T2S) are also impaired for a surfactant. When the tolC and lspF mutants were grown next to each other, the lsp mutant secreted surfactant, suggesting that TolC and T2S conjoin to mediate surfactant secretion, with one being the conduit for surfactant export and the other the exporter of a molecule that is required for induction or maturation of surfactant synthesis/secretion. Although the surfactant was not required for the extracellular growth, intracellular infection, and intrapulmonary survival of L. pneumophila, it exhibited antimicrobial activity toward seven other species of Legionella but not toward various non-Legionella species. These data suggest that the surfactant provides L. pneumophila with a selective advantage over other legionellae in the natural environment.
Figures

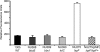


Similar articles
-
Surface translocation by Legionella pneumophila: a form of sliding motility that is dependent upon type II protein secretion.J Bacteriol. 2009 Mar;191(5):1537-46. doi: 10.1128/JB.01531-08. Epub 2008 Dec 29. J Bacteriol. 2009. PMID: 19114479 Free PMC article.
-
Legionella pneumophila type II protein secretion promotes virulence in the A/J mouse model of Legionnaires' disease pneumonia.Infect Immun. 2004 Jan;72(1):310-21. doi: 10.1128/IAI.72.1.310-321.2004. Infect Immun. 2004. PMID: 14688110 Free PMC article.
-
The type II protein secretion system of Legionella pneumophila promotes growth at low temperatures.J Bacteriol. 2004 Jun;186(12):3712-20. doi: 10.1128/JB.186.12.3712-3720.2004. J Bacteriol. 2004. PMID: 15175284 Free PMC article.
-
Pathogenicity of Legionella pneumophila.Int J Med Microbiol. 2001 Nov;291(5):331-43. doi: 10.1078/1438-4221-00139. Int J Med Microbiol. 2001. PMID: 11727817 Review.
-
Type II secretion and Legionella virulence.Curr Top Microbiol Immunol. 2013;376:81-102. doi: 10.1007/82_2013_339. Curr Top Microbiol Immunol. 2013. PMID: 23900831 Review.
Cited by
-
Biofilms: the stronghold of Legionella pneumophila.Int J Mol Sci. 2013 Oct 31;14(11):21660-75. doi: 10.3390/ijms141121660. Int J Mol Sci. 2013. PMID: 24185913 Free PMC article. Review.
-
Subsurface hydrocarbon degradation strategies in low- and high-sulfate coal seam communities identified with activity-based metagenomics.NPJ Biofilms Microbiomes. 2022 Feb 17;8(1):7. doi: 10.1038/s41522-022-00267-2. NPJ Biofilms Microbiomes. 2022. PMID: 35177633 Free PMC article.
-
Legionella pneumophila Cas2 Promotes the Expression of Small Heat Shock Protein C2 That Is Required for Thermal Tolerance and Optimal Intracellular Infection.Infect Immun. 2022 Oct 20;90(10):e0036922. doi: 10.1128/iai.00369-22. Epub 2022 Sep 8. Infect Immun. 2022. PMID: 36073935 Free PMC article.
-
Peptidyl-Prolyl-cis/trans-Isomerases Mip and PpiB of Legionella pneumophila Contribute to Surface Translocation, Growth at Suboptimal Temperature, and Infection.Infect Immun. 2018 Dec 19;87(1):e00939-17. doi: 10.1128/IAI.00939-17. Print 2019 Jan. Infect Immun. 2018. PMID: 30323027 Free PMC article.
-
Informatic analysis reveals Legionella as a source of novel natural products.Synth Syst Biotechnol. 2016 Feb 5;1(2):130-136. doi: 10.1016/j.synbio.2015.12.001. eCollection 2016 Jun. Synth Syst Biotechnol. 2016. PMID: 29062936 Free PMC article.
References
-
- Agusti G., Astola O., Rodriguez-Guell E., Julian E., Luquin M. 2008. Surface spreading motility shown by a group of phylogenetically related, rapidly growing pigmented mycobacteria suggests that motility is a common property of mycobacterial species but is restricted to smooth colonies. J. Bacteriol. 190:6894–6902 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

