The ageing systemic milieu negatively regulates neurogenesis and cognitive function
- PMID: 21886162
- PMCID: PMC3170097
- DOI: 10.1038/nature10357
The ageing systemic milieu negatively regulates neurogenesis and cognitive function
Abstract
In the central nervous system, ageing results in a precipitous decline in adult neural stem/progenitor cells and neurogenesis, with concomitant impairments in cognitive functions. Interestingly, such impairments can be ameliorated through systemic perturbations such as exercise. Here, using heterochronic parabiosis we show that blood-borne factors present in the systemic milieu can inhibit or promote adult neurogenesis in an age-dependent fashion in mice. Accordingly, exposing a young mouse to an old systemic environment or to plasma from old mice decreased synaptic plasticity, and impaired contextual fear conditioning and spatial learning and memory. We identify chemokines--including CCL11 (also known as eotaxin)--the plasma levels of which correlate with reduced neurogenesis in heterochronic parabionts and aged mice, and the levels of which are increased in the plasma and cerebrospinal fluid of healthy ageing humans. Lastly, increasing peripheral CCL11 chemokine levels in vivo in young mice decreased adult neurogenesis and impaired learning and memory. Together our data indicate that the decline in neurogenesis and cognitive impairments observed during ageing can be in part attributed to changes in blood-borne factors.
Conflict of interest statement
Figures

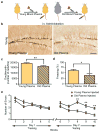
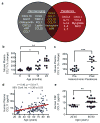
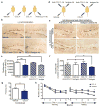
Comment in
-
Ageing: Blood ties.Nature. 2011 Aug 31;477(7362):41-2. doi: 10.1038/477041a. Nature. 2011. PMID: 21886154 No abstract available.
-
Ageing: ageing, it's in the blood.Nat Rev Neurosci. 2011 Sep 20;12(10):547. doi: 10.1038/nrn3116. Nat Rev Neurosci. 2011. PMID: 21931332 No abstract available.
Similar articles
-
Overcoming the aging systemic milieu to restore neural stem cell function.Rejuvenation Res. 2011 Dec;14(6):681-4. doi: 10.1089/rej.2011.1301. Rejuvenation Res. 2011. PMID: 22175510
-
β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis.Nat Med. 2015 Aug;21(8):932-7. doi: 10.1038/nm.3898. Epub 2015 Jul 6. Nat Med. 2015. PMID: 26147761 Free PMC article.
-
Young bone marrow transplantation preserves learning and memory in old mice.Commun Biol. 2019 Feb 20;2:73. doi: 10.1038/s42003-019-0298-5. eCollection 2019. Commun Biol. 2019. PMID: 30820468 Free PMC article.
-
The systemic environment: at the interface of aging and adult neurogenesis.Cell Tissue Res. 2018 Jan;371(1):105-113. doi: 10.1007/s00441-017-2715-8. Epub 2017 Nov 9. Cell Tissue Res. 2018. PMID: 29124393 Free PMC article. Review.
-
An emerging role for eotaxins in neurodegenerative disease.Clin Immunol. 2018 Apr;189:29-33. doi: 10.1016/j.clim.2016.09.010. Epub 2016 Sep 21. Clin Immunol. 2018. PMID: 27664933 Review.
Cited by
-
Maternal dysbiosis produces long-lasting behavioral changes in offspring.Mol Psychiatry. 2024 Oct 23. doi: 10.1038/s41380-024-02794-0. Online ahead of print. Mol Psychiatry. 2024. PMID: 39443733
-
A simple cell proliferation assay and the inflammatory protein content show significant differences in human plasmas from young and old subjects.Front Bioeng Biotechnol. 2024 Sep 9;12:1408499. doi: 10.3389/fbioe.2024.1408499. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39351061 Free PMC article.
-
Effects of obesogenic diet and 17β-estradiol in female mice with APOE 3/3, 3/4, and 4/4 genotypes.Front Aging Neurosci. 2024 Sep 13;16:1415072. doi: 10.3389/fnagi.2024.1415072. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39347015 Free PMC article.
-
Limitations and potential strategies of immune checkpoint blockade in age-related neurodegenerative disorders.J Physiol Sci. 2024 Sep 23;74(1):46. doi: 10.1186/s12576-024-00933-4. J Physiol Sci. 2024. PMID: 39313800 Free PMC article. Review.
-
Hallmarks of aging in age-related macular degeneration and age-related neurological disorders: novel insights into common mechanisms and clinical relevance.Eye (Lond). 2024 Sep 17. doi: 10.1038/s41433-024-03341-5. Online ahead of print. Eye (Lond). 2024. PMID: 39289517 Review.
References
-
- Gage FH. Mammalian neural stem cells. Science. 2000;287 (5457):1433–1438. - PubMed
-
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41 (5):683–686. - PubMed
-
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132 (4):645–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007290/AI/NIAID NIH HHS/United States
- F31 AG034045-03/AG/NIA NIH HHS/United States
- R01 MH078194/MH/NIMH NIH HHS/United States
- DP1 OD000392-02/OD/NIH HHS/United States
- F31 AG034045-02/AG/NIA NIH HHS/United States
- DP1 OD000392-04/OD/NIH HHS/United States
- DP1 OD000392-01/OD/NIH HHS/United States
- DP1 OD000392-05/OD/NIH HHS/United States
- F31 AG034045-01/AG/NIA NIH HHS/United States
- R01 AG027505-02/AG/NIA NIH HHS/United States
- 1 F31 AG034045-01/AG/NIA NIH HHS/United States
- R01AG027505/AG/NIA NIH HHS/United States
- R01 AG027505-01A1/AG/NIA NIH HHS/United States
- P30AG08017/AG/NIA NIH HHS/United States
- DP1 OD000392/OD/NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- R01 AG027505-05/AG/NIA NIH HHS/United States
- R01 AG027505/AG/NIA NIH HHS/United States
- R01 AR056849/AR/NIAMS NIH HHS/United States
- 1 F31 NS066676-01A1/NS/NINDS NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- DP1 OD000392-03/OD/NIH HHS/United States
- R01 AG027505-04/AG/NIA NIH HHS/United States
- F31 NS066676/NS/NINDS NIH HHS/United States
- R01 AG027505-03/AG/NIA NIH HHS/United States
- F31 AG034045/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

