Sensing damage by the NLRP3 inflammasome
- PMID: 21884174
- PMCID: PMC3170135
- DOI: 10.1111/j.1600-065X.2011.01043.x
Sensing damage by the NLRP3 inflammasome
Abstract
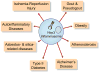
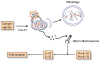
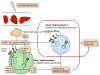
The NLRP3 inflammasome is activated in response to a variety of signals that are indicative of damage to the host including tissue damage, metabolic stress, and infection. Upon activation, the NLRP3 inflammasome serves as a platform for activation of the cysteine protease caspase-1, which leads to the processing and secretion of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18. Dysregulated NLRP3 inflammasome activation is associated with both heritable and acquired inflammatory diseases. Here, we review new insights into the mechanism of NLRP3 inflammasome activation and its role in disease pathogenesis.
© 2011 John Wiley & Sons A/S.
Figures
Similar articles
-
Molecular mechanism of NLRP3 inflammasome activation.J Clin Immunol. 2010 Sep;30(5):628-31. doi: 10.1007/s10875-010-9440-3. Epub 2010 Jun 30. J Clin Immunol. 2010. PMID: 20589420 Review.
-
NLRP3 inflammasomes link inflammation and metabolic disease.Trends Immunol. 2011 Aug;32(8):373-9. doi: 10.1016/j.it.2011.05.004. Epub 2011 Jul 4. Trends Immunol. 2011. PMID: 21733753 Free PMC article. Review.
-
The inflammasome: an integrated view.Immunol Rev. 2011 Sep;243(1):136-51. doi: 10.1111/j.1600-065X.2011.01046.x. Immunol Rev. 2011. PMID: 21884173 Review.
-
Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection.Proc Natl Acad Sci U S A. 2013 Oct 29;110(44):17963-8. doi: 10.1073/pnas.1312571110. Epub 2013 Oct 14. Proc Natl Acad Sci U S A. 2013. PMID: 24127597 Free PMC article.
-
The NLRP3 inflammasome in kidney disease and autoimmunity.Nephrology (Carlton). 2016 Sep;21(9):736-44. doi: 10.1111/nep.12785. Nephrology (Carlton). 2016. PMID: 27011059 Review.
Cited by
-
Commentary: APOE e4 Genotype Predicts Severe COVID-19 in the UK Biobank Community Cohort.Front Immunol. 2020 Sep 15;11:1939. doi: 10.3389/fimmu.2020.01939. eCollection 2020. Front Immunol. 2020. PMID: 33042114 Free PMC article. No abstract available.
-
The Regulatory Role of Rolipram on Inflammatory Mediators and Cholinergic/Adrenergic Stimulation-Induced Signals in Isolated Primary Mouse Submandibular Gland Cells.Mediators Inflamm. 2016;2016:3745961. doi: 10.1155/2016/3745961. Epub 2016 Apr 7. Mediators Inflamm. 2016. PMID: 27143817 Free PMC article.
-
Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal.Neuropsychopharmacology. 2017 Jan;42(1):46-61. doi: 10.1038/npp.2016.102. Epub 2016 Jun 20. Neuropsychopharmacology. 2017. PMID: 27319971 Free PMC article. Review.
-
Cyclic Stretching Exacerbates Tendinitis by Enhancing NLRP3 Inflammasome Activity via F-Actin Depolymerization.Inflammation. 2018 Oct;41(5):1731-1743. doi: 10.1007/s10753-018-0816-5. Inflammation. 2018. PMID: 29951874
-
A novel scoring system for acute myeloid leukemia risk assessment based on the expression levels of six genes.Int J Mol Med. 2018 Sep;42(3):1495-1507. doi: 10.3892/ijmm.2018.3739. Epub 2018 Jun 21. Int J Mol Med. 2018. PMID: 29956722 Free PMC article.
References
-
- Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. - PubMed
-
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. - PubMed
-
- Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007;282:36321–36329. - PubMed
-
- Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

