Beyond corticosteroids: future prospects in the management of inflammation in COPD
- PMID: 21881145
- PMCID: PMC9584116
- DOI: 10.1183/09059180.00004211
Beyond corticosteroids: future prospects in the management of inflammation in COPD
Abstract
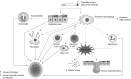
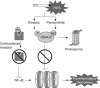
Inflammation plays a central role in the pathophysiology of chronic obstructive pulmonary disease (COPD). Exposure to cigarette smoke induces the recruitment of inflammatory cells in the airways and stimulates innate and adaptive immune mechanisms. Airway inflammation is involved in increased bronchial wall thickness, increased bronchial smooth muscle tone, mucus hypersecretion and loss of parenchymal elastic structures. Oxidative stress impairs tissue integrity, accelerates lung ageing and reduces the efficacy of corticosteroids by decreasing levels of histone deacetylase-2. Protease-antiprotease imbalance impairs tissues and is involved in inflammatory processes. Inflammation is also present in the pulmonary artery wall and at the systemic level in COPD patients, and may be involved in COPD-associated comorbidities. Proximal airways inflammation contributes to symptoms of chronic bronchitis while distal and parenchymal inflammation relates to airflow obstruction, emphysema and hyperinflation. Basal levels of airways and systemic inflammation are increased in frequent exacerbators. Inhaled corticosteroids are much less effective in COPD than in asthma, which relates to the intrinsically poor reversibility of COPD-related airflow obstruction and to molecular mechanisms of resistance relating to oxidative stress. Ongoing research aims at developing new drugs targeting more intimately COPD-specific mechanisms of inflammation, hypersecretion and tissue destruction and repair. Among new anti-inflammatory agents, phosphodiesterase-4 inhibitors have been the first to emerge.
Conflict of interest statement
N. Roche received fees for speaking, organising education, or consulting from Altana Pharma, Nycomed, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, MEDA, MSD-Chibret, Mundipharma, Novartis, Hoffman la Roche, Pfizer and Teva. He has received research grants from Nycomed and Boehringer Ingelheim. R. Marthan has received fees for speaking and travel to the ATS congress was funded by Nycomed. P. Berger received fees for speaking or consulting from Nycomed, Novartis, GlaxoSmithKline, AstraZeneca and Chiesi. He has received funds for research from Nycomed, Novartis and GlaxoSmithKline, and travel to the ERS and ATS congress was funded by Nycomed, Novartis, GlaxoSmithKline and Astra-Zeneca. A. Chambellan has received congress support from and/or served as a consultant for GlaxoSmithKline, Boehringer Ingelheim, Novartis and Nycomed. P. Chanez has provided consultancy services for Almirall, Boehringer Ingelheim, Centocor, GlaxoSmithKline, AstraZeneca, Novartis, Teva, Chiesi and Schering Plough; served on advisory boards for Almirall, Boehringer Ingelheim, Centocor, GlaxoSmithKline, AstraZeneca, Novartis, Teva, Chiesi and Schering Plough; received lecture fees from Almirall, Boehringer Ingelheim, Centocor, GlaxoSmithKline, AstraZeneca, Novartis, Teva, Chiesi and Schering Plough; and received industry-sponsored grants from Almirall, Boehringer Ingelheim, Centocor, GlaxoSmithKline, AstraZeneca, Novartis, Teva, Chiesi and Schering Plough. B. Aguilaniu has served as a consultant for Boehringer Ingelheim, Nycomed and Pfizer. P-Y. Brillet received fees for consulting from Nycomed and his travel to the 2010 ERS congress was funded by Nycomed. P-R. Burgel has received fees for speaking and consulting for Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Nycomed, AstraZeneca and Pfizer. P. Devillier has received fees for speaking, organising education, consulting research and congress support from AstraZeneca, Chiesi, GlaxoSmithKline, Novartis, Nycomed, Altana Pharma, Almirall, Boehringer Ingelheim, Stallergenes, Pfizer, Schering-Plough, and Merck Sharp and Dohme. R. Escamilla has served as a consultant for Boehringer Ingelheim, Chiesi, Novartis, Nycomed and Pfizer. R. Louis has served as a member of an advisory board for AstraZeneca, GlaxoSmithKline, Novartis, Nycomed, MSD and Chiesi, and received unrestricted research grants from AstraZeneca, GlaxoSmithKline and Novartis. J-F. Muir has received a fee for consulting from Nycomed, GlaxoSmithKline and Boehringer Ingelheim. T. Pérez has received fees for speaking at congresses from Chiesi, Novartis and Nycomed, fees for workshops by Chiesi and Nycomed, funding for academic research from Nycomed and Novartis, and is a consultant for AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Nycomed, Schering Plough and Pierre Fabre. His travel to ERS and CPLF congresses was funded by Chiesi, Nycomed, Boehringer Ingelheim and AstraZeneca. T. Similowski has received unrestricted research grants from Novartis and Maquet, lecture fees from Nycomed, Novartis, Boehringer Ingelheim, AstraZeneca, MSD and Medapharma, and has received honoraria from Nicomedia and Boehringer Ingelheim for organising education. He has received reimbursement for congresses expenses from Nycomed, Boehringer Ingelheim and AstraZeneca, and has received consulting fees from Nycomed, Novartis Boehringer Ingelheim, AstraZeneca and Pierre Fabre.
Figures
Similar articles
-
Influence of Inhaled Corticosteroids on Bronchial Inflammation and Pulmonary Function in Chronic Obstructive Pulmonary Disease with Moderate Obstruction.Adv Exp Med Biol. 2018;1047:41-52. doi: 10.1007/5584_2017_129. Adv Exp Med Biol. 2018. PMID: 29181828
-
Use of inhaled corticosteroids in COPD: improving efficacy.Expert Rev Respir Med. 2016;10(3):339-50. doi: 10.1586/17476348.2016.1151789. Expert Rev Respir Med. 2016. PMID: 26855301 Review.
-
Is there a role for systemic corticosteroids in the management of stable chronic obstructive pulmonary disease?Am J Respir Med. 2003;2(6):451-8. doi: 10.1007/BF03256672. Am J Respir Med. 2003. PMID: 14719984 Review.
-
Update on molecular mechanisms of corticosteroid resistance in chronic obstructive pulmonary disease.Pulm Pharmacol Ther. 2016 Apr;37:1-8. doi: 10.1016/j.pupt.2016.01.002. Epub 2016 Jan 22. Pulm Pharmacol Ther. 2016. PMID: 26805715 Review.
-
Distinguishing adult-onset asthma from COPD: a review and a new approach.Int J Chron Obstruct Pulmon Dis. 2014 Sep 9;9:945-62. doi: 10.2147/COPD.S46761. eCollection 2014. Int J Chron Obstruct Pulmon Dis. 2014. PMID: 25246782 Free PMC article. Review.
Cited by
-
Roles of histone deacetylases in epigenetic regulation: emerging paradigms from studies with inhibitors.Clin Epigenetics. 2012 Mar 12;4(1):5. doi: 10.1186/1868-7083-4-5. Clin Epigenetics. 2012. PMID: 22414492 Free PMC article.
-
In vitro anti-inflammatory effects of AZD8999, a novel bifunctional muscarinic acetylcholine receptor antagonist /β2-adrenoceptor agonist (MABA) compound in neutrophils from COPD patients.PLoS One. 2019 Jan 4;14(1):e0210188. doi: 10.1371/journal.pone.0210188. eCollection 2019. PLoS One. 2019. PMID: 30608978 Free PMC article.
-
Non-neuronal cholinergic system contributes to corticosteroid resistance in chronic obstructive pulmonary disease patients.Respir Res. 2016 Nov 8;17(1):145. doi: 10.1186/s12931-016-0467-8. Respir Res. 2016. PMID: 27825347 Free PMC article.
-
Roflumilast improves corticosteroid resistance COPD bronchial epithelial cells stimulated with toll like receptor 3 agonist.Respir Res. 2015 Feb 5;16(1):12. doi: 10.1186/s12931-015-0179-5. Respir Res. 2015. PMID: 25652132 Free PMC article.
-
Blood Eosinophils as Biomarkers to Drive Treatment Choices in Asthma and COPD.Curr Drug Targets. 2018;19(16):1882-1896. doi: 10.2174/1389450119666180212120012. Curr Drug Targets. 2018. PMID: 29437007 Free PMC article. Review.
References
-
- Calverley PM, Anderson JA, Celli B, et al. . Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356: 775–789. - PubMed
-
- Celli BR, Thomas NE, Anderson JA, et al. . Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med 2008; 178: 332–338. - PubMed
-
- Tashkin DP, Celli B, Senn S, et al. . A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359: 1543–1554. - PubMed
-
- Aubier M, Marthan R, Berger P, et al. . BPCO et inflammation: mise au point d‘un group d‘experts. Les mécanismes de l‘inflammation et du remodelage [COPD and inflammation: statement from a French expert group: inflammation and remodelling mechanisms]. Rev Mal Respir 2010; 27: 1254–1266. - PubMed
-
- Pérez T, Mal H, Aguilaniu B, et al. . BPCO et inflammation: mise au point d‘un group d‘experts. Les phénotypes en lien avec l‘inflammation [COPD and inflammation: statement from a French expert group. Phenotypes related to inflammation]. Rev Mal Respir 2011; 28: 192–215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
