A novel fluorescent histamine H(1) receptor antagonist demonstrates the advantage of using fluorescence correlation spectroscopy to study the binding of lipophilic ligands
- PMID: 21880035
- PMCID: PMC3372830
- DOI: 10.1111/j.1476-5381.2011.01640.x
A novel fluorescent histamine H(1) receptor antagonist demonstrates the advantage of using fluorescence correlation spectroscopy to study the binding of lipophilic ligands
Abstract
Background and purpose: Fluorescent ligands facilitate the study of ligand-receptor interactions at the level of single cells and individual receptors. Here, we describe a novel fluorescent histamine H(1) receptor antagonist (mepyramine-BODIPY630-650) and use it to monitor the membrane diffusion of the histamine H(1) receptor.
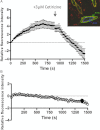
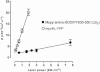
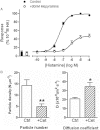
Experimental approach: The human histamine H(1) receptor fused to yellow fluorescent protein (YFP) was transiently expressed in CHO-K1 cells. The time course of binding of mepyramine-BODIPY630-650 to the H(1) receptor was determined by confocal microscopy. Additionally, fluorescence correlation spectroscopy (FCS) was used to characterize the diffusion coefficient of the H(1) receptor in cell membranes both directly (YFP fluorescence) and in its antagonist-bound state (with mepyramine-BODIPY630-650).
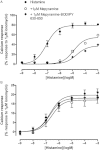
Key results: Mepyramine-BODIPY630-650 was a high-affinity antagonist at the histamine H(1) receptor. Specific membrane binding, in addition to significant intracellular uptake of the fluorescent ligand, was detected by confocal microscopy. However, FCS was able to quantify the receptor-specific binding in the membrane, as well as the diffusion coefficient of the antagonist-H(1) receptor-YFP complexes, which was significantly slower than when determined directly using YFP. FCS also detected specific binding of mepyramine-BODIPY630-650 to the endogenous H(1) receptor in HeLa cells.
Conclusions and implications: Mepyramine-BODIPY630-650 is a useful tool for localizing the H(1) receptor using confocal microscopy. However, its use in conjunction with FCS allows quantification of ligand binding at the membrane, as well as determining receptor diffusion in the absence of significant bleaching effects. Finally, these methods can be successfully extended to endogenously expressed untagged receptors in HeLa cells.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures


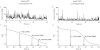

Similar articles
-
Mepyramine, a histamine H1 receptor inverse agonist, binds preferentially to a G protein-coupled form of the receptor and sequesters G protein.J Biol Chem. 2004 Aug 13;279(33):34431-9. doi: 10.1074/jbc.M400738200. Epub 2004 Jun 10. J Biol Chem. 2004. PMID: 15192105
-
Pharmacological characterization of GSK1004723, a novel, long-acting antagonist at histamine H(1) and H(3) receptors.Br J Pharmacol. 2011 Nov;164(6):1627-41. doi: 10.1111/j.1476-5381.2011.01285.x. Br J Pharmacol. 2011. PMID: 22022805 Free PMC article.
-
Characteristics of the binding of [3H]-mepyramine to intact human U373 MG astrocytoma cells: evidence for histamine-induced H1-receptor internalisation.Br J Pharmacol. 1995 Nov;116(6):2715-23. doi: 10.1111/j.1476-5381.1995.tb17232.x. Br J Pharmacol. 1995. PMID: 8590995 Free PMC article.
-
Ca2+ -dependent down-regulation of human histamine H1 receptors in Chinese hamster ovary cells.J Neurochem. 2018 Jan;144(1):68-80. doi: 10.1111/jnc.14245. Epub 2017 Nov 21. J Neurochem. 2018. PMID: 29063596
-
Delineation of receptor-ligand interactions at the human histamine H1 receptor by a combined approach of site-directed mutagenesis and computational techniques - or - how to bind the H1 receptor.Arch Pharm (Weinheim). 2005 Jun;338(5-6):248-59. doi: 10.1002/ardp.200400998. Arch Pharm (Weinheim). 2005. PMID: 15952243 Review.
Cited by
-
Fluorescent approaches for understanding interactions of ligands with G protein coupled receptors.Biochim Biophys Acta. 2014 Jan;1838(1 Pt A):15-33. doi: 10.1016/j.bbamem.2013.09.005. Epub 2013 Sep 18. Biochim Biophys Acta. 2014. PMID: 24055822 Free PMC article. Review.
-
Optimization of Peptide Linker-Based Fluorescent Ligands for the Histamine H1 Receptor.J Med Chem. 2022 Jun 23;65(12):8258-8288. doi: 10.1021/acs.jmedchem.2c00125. Epub 2022 Jun 3. J Med Chem. 2022. PMID: 35734860 Free PMC article.
-
CRISPR-Mediated Protein Tagging with Nanoluciferase to Investigate Native Chemokine Receptor Function and Conformational Changes.Cell Chem Biol. 2020 May 21;27(5):499-510.e7. doi: 10.1016/j.chembiol.2020.01.010. Epub 2020 Feb 12. Cell Chem Biol. 2020. PMID: 32053779 Free PMC article.
-
Quantifying intracellular dynamics using fluorescence fluctuation spectroscopy.Protoplasma. 2014 Mar;251(2):307-16. doi: 10.1007/s00709-013-0602-z. Epub 2014 Jan 14. Protoplasma. 2014. PMID: 24420265 Review.
-
The use of fluorescence correlation spectroscopy to characterize the molecular mobility of fluorescently labelled G protein-coupled receptors.Biochem Soc Trans. 2016 Apr 15;44(2):624-9. doi: 10.1042/BST20150285. Biochem Soc Trans. 2016. PMID: 27068980 Free PMC article. Review.
References
-
- Bakker RA, Timmerman H, Leurs R. Histamine receptors: specific ligands, receptor biochemistry and signal transduction. Clin Allergy Immunol. 2002;17:27–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

