Synthesis and characterization of high-affinity 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene-labeled fluorescent ligands for human β-adrenoceptors
- PMID: 21870877
- PMCID: PMC3188295
- DOI: 10.1021/jm2008562
Synthesis and characterization of high-affinity 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene-labeled fluorescent ligands for human β-adrenoceptors
Abstract
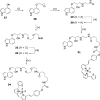
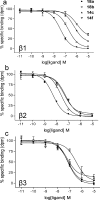
The growing practice of exploiting noninvasive fluorescence-based techniques to study G protein-coupled receptor pharmacology at the single cell and single molecule level demands the availability of high-quality fluorescent ligands. To this end, this study evaluated a new series of red-emitting ligands for the human β-adrenoceptor family. Upon the basis of the orthosteric ligands propranolol, alprenolol, and pindolol, the synthesized linker-modified congeners were coupled to the commercially available fluorophore BODIPY 630/650-X. This yielded high-affinity β-adrenoceptor fluorescent ligands for both the propranolol and alprenolol derivatives; however, the pindolol-based products displayed lower affinity. A fluorescent diethylene glycol linked propranolol derivative (18a) had the highest affinity (log K(D) of -9.53 and -8.46 as an antagonist of functional β2- and β1-mediated responses, respectively). Imaging studies with this compound further confirmed that it can be employed to selectively label the human β2-adrenoceptor in single living cells, with receptor-associated binding prevented by preincubation with the nonfluorescent β2-selective antagonist 3-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol (ICI 118551) ( J. Cardiovasc. Pharmacol.1983, 5, 430-437. ).
Figures



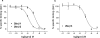

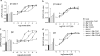

Similar articles
-
The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors.Br J Pharmacol. 2005 Feb;144(3):317-22. doi: 10.1038/sj.bjp.0706048. Br J Pharmacol. 2005. PMID: 15655528 Free PMC article.
-
Detection of the secondary, low-affinity β1 -adrenoceptor site in living cells using the fluorescent CGP 12177 derivative BODIPY-TMR-CGP.Br J Pharmacol. 2014 Dec;171(23):5431-45. doi: 10.1111/bph.12858. Br J Pharmacol. 2014. PMID: 25052258 Free PMC article.
-
Intrinsic sympathomimetic activity of (-)-pindolol mediated through a (-)-propranolol-resistant site of the beta1-adrenoceptor in human atrium and recombinant receptors.Naunyn Schmiedebergs Arch Pharmacol. 2003 Dec;368(6):496-503. doi: 10.1007/s00210-003-0835-z. Epub 2003 Nov 8. Naunyn Schmiedebergs Arch Pharmacol. 2003. PMID: 14608456
-
Human heart beta-adrenoceptors: beta1-adrenoceptor diversification through 'affinity states' and polymorphism.Clin Exp Pharmacol Physiol. 2007 Oct;34(10):1020-8. doi: 10.1111/j.1440-1681.2007.04730.x. Clin Exp Pharmacol Physiol. 2007. PMID: 17714089 Review.
-
Fluorescent ligands for adenosine receptors.Bioorg Med Chem Lett. 2013 Jan 1;23(1):26-36. doi: 10.1016/j.bmcl.2012.10.112. Epub 2012 Nov 5. Bioorg Med Chem Lett. 2013. PMID: 23200243 Free PMC article. Review.
Cited by
-
Synthesis of BODIPY derivatives substituted with various bioconjugatable linker groups: a construction kit for fluorescent labeling of receptor ligands.J Fluoresc. 2014 Jan;24(1):213-30. doi: 10.1007/s10895-013-1289-4. Epub 2013 Sep 20. J Fluoresc. 2014. PMID: 24052460
-
Fluorescent approaches for understanding interactions of ligands with G protein coupled receptors.Biochim Biophys Acta. 2014 Jan;1838(1 Pt A):15-33. doi: 10.1016/j.bbamem.2013.09.005. Epub 2013 Sep 18. Biochim Biophys Acta. 2014. PMID: 24055822 Free PMC article. Review.
-
Discovery of Quinazoline-Based Fluorescent Probes to α1-Adrenergic Receptors.ACS Med Chem Lett. 2015 Mar 30;6(5):502-6. doi: 10.1021/ml5004298. eCollection 2015 May 14. ACS Med Chem Lett. 2015. PMID: 26005522 Free PMC article.
-
Kinetic analysis of antagonist-occupied adenosine-A3 receptors within membrane microdomains of individual cells provides evidence of receptor dimerization and allosterism.FASEB J. 2014 Oct;28(10):4211-22. doi: 10.1096/fj.13-247270. Epub 2014 Jun 26. FASEB J. 2014. PMID: 24970394 Free PMC article.
-
Impact of salt stress, cell death, and autophagy on peroxisomes: quantitative and morphological analyses using small fluorescent probe N-BODIPY.Sci Rep. 2017 Feb 1;7:39069. doi: 10.1038/srep39069. Sci Rep. 2017. PMID: 28145408 Free PMC article.
References
-
- Bilski A. J.; Halliday S. E.; Fitzgerald J. D.; Wale J. L. The pharmacology of a beta 2-selective adrenoceptor antagonist (ICI 118,551). J. Cardiovasc. Pharmacol. 1983, 5, 430–437. - PubMed
-
- Jacoby E.; Bouhelal R.; Gerspacher M.; Seuwen K. The 7 TM G-protein coupled receptor target family. ChemMedChem 2006, 1, 760–782. - PubMed
-
- Bylund D. B.; Bond R. A.; Eikenburg D. C.; J. Hieble P. J. Hills R.; Minneman K. P.; Parra S.. Beta-adrenoceptors. http://www.iuphar-db.org/DATABASE/FamilyMenuForward?familyId=4.
-
- Michel M. C.; Insel P. A.. Adrenergic receptors in clinical medicine. In The Receptors: The Adrenergic Receptors: In the 21st Century; Perez D. M., Ed.; Humana Press: New York, 2007; pp 129–147.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

