Role of Survivin in cytokinesis revealed by a separation-of-function allele
- PMID: 21865602
- PMCID: PMC3192858
- DOI: 10.1091/mbc.E11-06-0569
Role of Survivin in cytokinesis revealed by a separation-of-function allele
Abstract
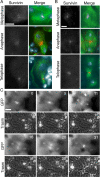
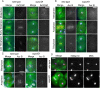
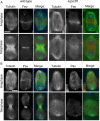
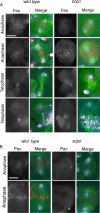
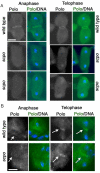
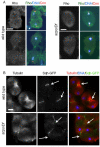
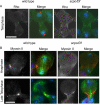
The chromosomal passenger complex (CPC), containing Aurora B kinase, Inner Centromere Protein, Survivin, and Borealin, regulates chromosome condensation and interaction between kinetochores and microtubules at metaphase, then relocalizes to midzone microtubules at anaphase and regulates central spindle organization and cytokinesis. However, the precise role(s) played by the CPC in anaphase have been obscured by its prior functions in metaphase. Here we identify a missense allele of Drosophila Survivin that allows CPC localization and function during metaphase but not cytokinesis. Analysis of mutant cells showed that Survivin is essential to target the CPC and the mitotic kinesin-like protein 1 orthologue Pavarotti (Pav) to the central spindle and equatorial cell cortex during anaphase in both larval neuroblasts and spermatocytes. Survivin also enabled localization of Polo kinase and Rho at the equatorial cortex in spermatocytes, critical for contractile ring assembly. In neuroblasts, in contrast, Survivin function was not required for localization of Rho, Polo, or Myosin II to a broad equatorial cortical band but was required for Myosin II to transition to a compact, fully constricted ring. Analysis of this "separation-of-function" allele demonstrates the direct role of Survivin and the CPC in cytokinesis and highlights striking differences in regulation of cytokinesis in different cell systems.
Figures


Similar articles
-
Australin: a chromosomal passenger protein required specifically for Drosophila melanogaster male meiosis.J Cell Biol. 2008 Feb 11;180(3):521-35. doi: 10.1083/jcb.200708072. J Cell Biol. 2008. PMID: 18268101 Free PMC article.
-
Dynactin targets Pavarotti-KLP to the central spindle during anaphase and facilitates cytokinesis in Drosophila S2 cells.J Cell Sci. 2006 Nov 1;119(Pt 21):4431-41. doi: 10.1242/jcs.03204. Epub 2006 Oct 17. J Cell Sci. 2006. PMID: 17046997
-
Asymmetrically dividing Drosophila neuroblasts utilize two spatially and temporally independent cytokinesis pathways.Nat Commun. 2015 Mar 20;6:6551. doi: 10.1038/ncomms7551. Nat Commun. 2015. PMID: 25791062 Free PMC article.
-
[Role of survivin in mitosis].Postepy Biochem. 2007;53(1):10-8. Postepy Biochem. 2007. PMID: 17718383 Review. Polish.
-
Changing places: Chromosomal Passenger Complex relocation in early anaphase.Trends Cell Biol. 2022 Feb;32(2):165-176. doi: 10.1016/j.tcb.2021.09.008. Epub 2021 Oct 16. Trends Cell Biol. 2022. PMID: 34663523 Review.
Cited by
-
Overexpression of caudal type homeobox transcription factor 2 inhibits the growth of the MGC-803 human gastric cancer cell line in vivo.Mol Med Rep. 2015 Jul;12(1):905-12. doi: 10.3892/mmr.2015.3413. Epub 2015 Mar 4. Mol Med Rep. 2015. PMID: 25738600 Free PMC article.
-
The ultrastructural organization of actin and myosin II filaments in the contractile ring: new support for an old model of cytokinesis.Mol Biol Cell. 2017 Mar 1;28(5):613-623. doi: 10.1091/mbc.E16-06-0466. Epub 2017 Jan 5. Mol Biol Cell. 2017. PMID: 28057763 Free PMC article.
-
The chromosomal passenger protein birc5b organizes microfilaments and germ plasm in the zebrafish embryo.PLoS Genet. 2013 Apr;9(4):e1003448. doi: 10.1371/journal.pgen.1003448. Epub 2013 Apr 18. PLoS Genet. 2013. PMID: 23637620 Free PMC article.
-
Drosophila Doublefault protein coordinates multiple events during male meiosis by controlling mRNA translation.Development. 2019 Nov 18;146(22):dev183053. doi: 10.1242/dev.183053. Development. 2019. PMID: 31645358 Free PMC article.
-
The Ins and Outs of Aurora B Inner Centromere Localization.Front Cell Dev Biol. 2017 Dec 22;5:112. doi: 10.3389/fcell.2017.00112. eCollection 2017. Front Cell Dev Biol. 2017. PMID: 29312936 Free PMC article. Review.
References
-
- Beardmore VA, Ahonen LJ, Gorbsky GJ, Kallio MJ. Survivin dynamics increases at centromeres during G2/M phase transition and is regulated by microtubule-attachment and Aurora B kinase activity. J Cell Sci. 2004;117:4033–4042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

