Advanced glycation end products (AGEs) induce apoptosis via a novel pathway: involvement of Ca2+ mediated by interleukin-8 protein
- PMID: 21862577
- PMCID: PMC3186367
- DOI: 10.1074/jbc.M111.279190
Advanced glycation end products (AGEs) induce apoptosis via a novel pathway: involvement of Ca2+ mediated by interleukin-8 protein
Abstract
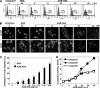
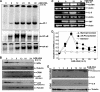
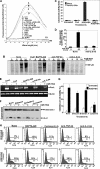
Advanced glycation end products (AGEs) accumulate in diabetic patients due to high blood glucose levels and cause multiple deleterious effects. In this study, we provide evidence that the AGE increased cell death, one such deleterious effect. Methyl glyoxal-coupled human serum albumin (AGE-HSA) induced transcription factors such as NF-κB, NF-AT, and AP-1. AGE acts through its cell surface receptor, RAGE, and degranulates vesicular contents including interleukin-8 (IL-8). The number of RAGEs, as well as the amount of NF-κB activation, is low, but the cell death is higher in neuronal cells upon AGE treatment. Degranulated IL-8 acts through its receptors, IL-8Rs, and induces sequential events in cells: increase in intracellular Ca(2+), activation of calcineurin, dephosphorylation of cytoplasmic NF-AT, nuclear translocation of NF-AT, and expression of FasL. Expressed FasL increases activity of caspases and induces cell death. Although AGE increases the amount of reactive oxygen intermediate, accompanying cell death is not dependent upon reactive oxygen intermediate. AGE induces autophagy, which partially protects cells from cell death. A novel mechanism of AGE-mediated cell death in different cell types, especially in neuronal cells where it is an early event, is provided here. Thus, this study may be important in several age-related neuronal diseases where AGE-induced apoptosis is observed because of high amounts of AGE.
Figures




Similar articles
-
Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes.Rheumatology (Oxford). 2011 May;50(5):838-51. doi: 10.1093/rheumatology/keq380. Epub 2010 Dec 20. Rheumatology (Oxford). 2011. PMID: 21172926 Free PMC article.
-
Advanced Glycation End Products (AGE) Potently Induce Autophagy through Activation of RAF Protein Kinase and Nuclear Factor κB (NF-κB).J Biol Chem. 2016 Jan 15;291(3):1481-91. doi: 10.1074/jbc.M115.667576. Epub 2015 Nov 19. J Biol Chem. 2016. PMID: 26586913 Free PMC article.
-
Advanced glycation end-products increase IL-6 and ICAM-1 expression via RAGE, MAPK and NF-κB pathways in human gingival fibroblasts.J Periodontal Res. 2018 Jun;53(3):334-344. doi: 10.1111/jre.12518. Epub 2017 Nov 30. J Periodontal Res. 2018. PMID: 29193068
-
Late phase activation of nuclear transcription factor kappaB by doxorubicin is mediated by interleukin-8 and induction of apoptosis via FasL.Breast Cancer Res Treat. 2010 Apr;120(3):671-83. doi: 10.1007/s10549-009-0493-z. Epub 2009 Aug 2. Breast Cancer Res Treat. 2010. Retraction in: Breast Cancer Res Treat. 2013 Apr;138(3):977. doi: 10.1007/s10549-013-2482-5 PMID: 19649704 Retracted.
-
Fas ligand induces cell-autonomous NF-kappaB activation and interleukin-8 production by a mechanism distinct from that of tumor necrosis factor-alpha.J Biol Chem. 2004 Nov 5;279(45):46415-23. doi: 10.1074/jbc.M403226200. Epub 2004 Aug 26. J Biol Chem. 2004. PMID: 15337758
Cited by
-
Advanced Glycation End Products Increase Salivary Gland Hypofunction in d-Galactose-Induced Aging Rats and Its Prevention by Physical Exercise.Curr Issues Mol Biol. 2021 Nov 19;43(3):2059-2067. doi: 10.3390/cimb43030142. Curr Issues Mol Biol. 2021. PMID: 34889900 Free PMC article.
-
AGE-RAGE axis culminates into multiple pathogenic processes: a central road to neurodegeneration.Front Mol Neurosci. 2023 May 17;16:1155175. doi: 10.3389/fnmol.2023.1155175. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37266370 Free PMC article. Review.
-
Impaired osteogenic differentiation and enhanced cellular receptor of advanced glycation end products sensitivity in patients with type 2 diabetes.J Bone Miner Metab. 2017 Nov;35(6):631-641. doi: 10.1007/s00774-016-0800-9. Epub 2016 Nov 21. J Bone Miner Metab. 2017. PMID: 27873077
-
Low-grade inflammation in survivors of childhood cancer and testicular cancer and its association with hypogonadism and metabolic risk factors.BMC Cancer. 2022 Feb 9;22(1):157. doi: 10.1186/s12885-022-09253-5. BMC Cancer. 2022. PMID: 35135482 Free PMC article.
-
Advanced Glycation End Products and Risks for Chronic Diseases: Intervening Through Lifestyle Modification.Am J Lifestyle Med. 2017 May 15;13(4):384-404. doi: 10.1177/1559827617708991. eCollection 2019 Jul-Aug. Am J Lifestyle Med. 2017. PMID: 31285723 Free PMC article.
References
-
- Franceschi C., Bonafè M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. (2000) Ann. N.Y. Acad. Sci. 908, 244–254 - PubMed
-
- Khuhawar M. Y., Kandhro A. J., Khand F. D. (2006) Anal. Lett. 39, 2205–2215
-
- Lapolla A., Flamini R., Dalla Vedova A., Senesi A., Reitano R., Fedele D., Basso E., Seraglia R., Traldi P. (2003) Clin. Chem. Lab. Med. 41, 1166–1173 - PubMed
-
- Odani H., Shinzato T., Matsumoto Y., Usami J., Maeda K. (1999) Biochem. Biophys. Res. Commun. 256, 89–93 - PubMed
-
- Singh R., Barden A., Mori T., Beilin L. (2001) Diabetologia 44, 129–146 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

