Gamma-tubulin is required for bipolar spindle assembly and for proper kinetochore microtubule attachments during prometaphase I in Drosophila oocytes
- PMID: 21852952
- PMCID: PMC3154956
- DOI: 10.1371/journal.pgen.1002209
Gamma-tubulin is required for bipolar spindle assembly and for proper kinetochore microtubule attachments during prometaphase I in Drosophila oocytes
Abstract
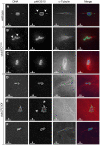
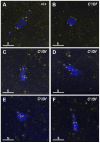
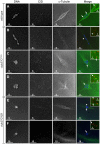
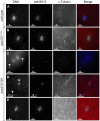
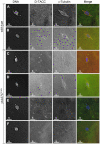
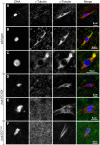
In many animal species the meiosis I spindle in oocytes is anastral and lacks centrosomes. Previous studies of Drosophila oocytes failed to detect the native form of the germline-specific γ-tubulin (γTub37C) in meiosis I spindles, and genetic studies have yielded conflicting data regarding the role of γTub37C in the formation of bipolar spindles at meiosis I. Our examination of living and fixed oocytes carrying either a null allele or strong missense mutation in the γtub37C gene demonstrates a role for γTub37C in the positioning of the oocyte nucleus during late prophase, as well as in the formation and maintenance of bipolar spindles in Drosophila oocytes. Prometaphase I spindles in γtub37C mutant oocytes showed wide, non-tapered spindle poles and disrupted positioning. Additionally, chromosomes failed to align properly on the spindle and showed morphological defects. The kinetochores failed to properly co-orient and often lacked proper attachments to the microtubule bundles, suggesting that γTub37C is required to stabilize kinetochore microtubule attachments in anastral spindles. Although spindle bipolarity was sometimes achieved by metaphase I in both γtub37C mutants, the resulting chromosome masses displayed highly disrupted chromosome alignment. Therefore, our data conclusively demonstrate a role for γTub37C in both the formation of the anastral meiosis I spindle and in the proper attachment of kinetochore microtubules. Finally, multispectral imaging demonstrates the presences of native γTub37C along the length of wild-type meiosis I spindles.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Anastral spindle assembly and γ-tubulin in Drosophila oocytes.BMC Cell Biol. 2011 Jan 5;12:1. doi: 10.1186/1471-2121-12-1. BMC Cell Biol. 2011. PMID: 21208439 Free PMC article.
-
Assembly pathway of the anastral Drosophila oocyte meiosis I spindle.J Cell Sci. 2005 Apr 15;118(Pt 8):1745-55. doi: 10.1242/jcs.02304. Epub 2005 Mar 29. J Cell Sci. 2005. PMID: 15797926 Free PMC article.
-
Intra-oocyte localization of MAD2 and its relationship with kinetochores, microtubules, and chromosomes in rat oocytes during meiosis.Biol Reprod. 2004 Sep;71(3):740-8. doi: 10.1095/biolreprod.104.028282. Epub 2004 Apr 28. Biol Reprod. 2004. PMID: 15115722
-
Oocyte Meiotic Spindle Assembly and Function.Curr Top Dev Biol. 2016;116:65-98. doi: 10.1016/bs.ctdb.2015.11.031. Epub 2016 Jan 23. Curr Top Dev Biol. 2016. PMID: 26970614 Free PMC article. Review.
-
The chromosomal basis of meiotic acentrosomal spindle assembly and function in oocytes.Chromosoma. 2017 Jun;126(3):351-364. doi: 10.1007/s00412-016-0618-1. Epub 2016 Nov 11. Chromosoma. 2017. PMID: 27837282 Free PMC article. Review.
Cited by
-
Nondisjunctional segregations in Drosophila female meiosis I are preceded by homolog malorientation at metaphase arrest.Genetics. 2013 Feb;193(2):443-51. doi: 10.1534/genetics.112.146241. Epub 2012 Dec 5. Genetics. 2013. PMID: 23222652 Free PMC article.
-
γ-Tubulin⁻γ-Tubulin Interactions as the Basis for the Formation of a Meshwork.Int J Mol Sci. 2018 Oct 19;19(10):3245. doi: 10.3390/ijms19103245. Int J Mol Sci. 2018. PMID: 30347727 Free PMC article. Review.
-
Centrosomal and Non-Centrosomal Microtubule-Organizing Centers (MTOCs) in Drosophila melanogaster.Cells. 2018 Aug 28;7(9):121. doi: 10.3390/cells7090121. Cells. 2018. PMID: 30154378 Free PMC article. Review.
-
Comparative Cytology of Female Meiosis I Among Drosophila Species.G3 (Bethesda). 2020 May 4;10(5):1765-1774. doi: 10.1534/g3.120.400867. G3 (Bethesda). 2020. PMID: 32217631 Free PMC article.
-
Metaphase Spindle Assembly.Biology (Basel). 2017 Feb 3;6(1):8. doi: 10.3390/biology6010008. Biology (Basel). 2017. PMID: 28165376 Free PMC article. Review.
References
-
- Raynaud-Messina B, Merdes A. Gamma-tubulin complexes and microtubule organization. Curr Opin Cell Biol. 2007;19:24–30. - PubMed
-
- Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130:484–498. - PubMed
-
- Barrett SL, Albertini DF. Allocation of gamma-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte. Biol Reprod. 2007;76:949–957. - PubMed
-
- Combelles CM, Albertini DF. Microtubule patterning during meiotic maturation in mouse oocytes is determined by cell cycle-specific sorting and redistribution of gamma-tubulin. Dev Biol. 2001;239:281–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

