B cell repertoire analysis identifies new antigenic domains on glycoprotein B of human cytomegalovirus which are target of neutralizing antibodies
- PMID: 21852946
- PMCID: PMC3154849
- DOI: 10.1371/journal.ppat.1002172
B cell repertoire analysis identifies new antigenic domains on glycoprotein B of human cytomegalovirus which are target of neutralizing antibodies
Abstract
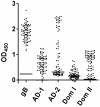
Human cytomegalovirus (HCMV), a herpesvirus, is a ubiquitously distributed pathogen that causes severe disease in immunosuppressed patients and infected newborns. Efforts are underway to prepare effective subunit vaccines and therapies including antiviral antibodies. However, current vaccine efforts are hampered by the lack of information on protective immune responses against HCMV. Characterizing the B-cell response in healthy infected individuals could aid in the design of optimal vaccines and therapeutic antibodies. To address this problem, we determined, for the first time, the B-cell repertoire against glycoprotein B (gB) of HCMV in different healthy HCMV seropositive individuals in an unbiased fashion. HCMV gB represents a dominant viral antigenic determinant for induction of neutralizing antibodies during infection and is also a component in several experimental HCMV vaccines currently being tested in humans. Our findings have revealed that the vast majority (>90%) of gB-specific antibodies secreted from B-cell clones do not have virus neutralizing activity. Most neutralizing antibodies were found to bind to epitopes not located within the previously characterized antigenic domains (AD) of gB. To map the target structures of these neutralizing antibodies, we generated a 3D model of HCMV gB and used it to identify surface exposed protein domains. Two protein domains were found to be targeted by the majority of neutralizing antibodies. Domain I, located between amino acids (aa) 133-343 of gB and domain II, a discontinuous domain, built from residues 121-132 and 344-438. Analysis of a larger panel of human sera from HCMV seropositive individuals revealed positivity rates of >50% against domain I and >90% against domain II, respectively. In accordance with previous nomenclature the domains were designated AD-4 (Dom II) and AD-5 (Dom I), respectively. Collectively, these data will contribute to optimal vaccine design and development of antibodies effective in passive immunization.
Conflict of interest statement
SP, NS, A-KW, HS, LM-P, MM, and THW are inventors on a patent application on the antibodies described. THW and MM received consulting fees from 4-Antibody AG.
Figures

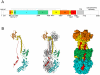
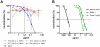
Similar articles
-
Identification of a neutralizing epitope within antigenic domain 5 of glycoprotein B of human cytomegalovirus.J Virol. 2015 Jan;89(1):361-72. doi: 10.1128/JVI.02393-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320309 Free PMC article.
-
Characterization of a discontinuous neutralizing epitope on glycoprotein B of human cytomegalovirus.J Virol. 2013 Aug;87(16):8927-39. doi: 10.1128/JVI.00434-13. Epub 2013 Jun 5. J Virol. 2013. PMID: 23740990 Free PMC article.
-
HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions.Proc Natl Acad Sci U S A. 2018 Jun 12;115(24):6267-6272. doi: 10.1073/pnas.1800177115. Epub 2018 Apr 30. Proc Natl Acad Sci U S A. 2018. PMID: 29712861 Free PMC article. Clinical Trial.
-
Human cytomegalovirus (HCMV) infection/re-infection: development of a protective HCMV vaccine.New Microbiol. 2019 Jan;42(1):1-20. Epub 2019 Jan 21. New Microbiol. 2019. PMID: 30671581 Review.
-
Cytomegalovirus Vaccines: Current Status and Future Prospects.Drugs. 2016 Nov;76(17):1625-1645. doi: 10.1007/s40265-016-0653-5. Drugs. 2016. PMID: 27882457 Free PMC article. Review.
Cited by
-
Multivalent cytomegalovirus glycoprotein B nucleoside modified mRNA vaccines did not demonstrate a greater antibody breadth.NPJ Vaccines. 2024 Feb 20;9(1):38. doi: 10.1038/s41541-024-00821-3. NPJ Vaccines. 2024. PMID: 38378950 Free PMC article.
-
Production of Cytomegalovirus Dense Bodies by Scalable Bioprocess Methods Maintains Immunogenicity and Improves Neutralizing Antibody Titers.J Virol. 2016 Oct 28;90(22):10133-10144. doi: 10.1128/JVI.00463-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27581989 Free PMC article.
-
Epitope-Specific Humoral Responses to Human Cytomegalovirus Glycoprotein-B Vaccine With MF59: Anti-AD2 Levels Correlate With Protection From Viremia.J Infect Dis. 2018 May 25;217(12):1907-1917. doi: 10.1093/infdis/jiy102. J Infect Dis. 2018. PMID: 29528415 Free PMC article.
-
A Luciferase Gene Driven by an Alphaherpesviral Promoter Also Responds to Immediate Early Antigens of the Betaherpesvirus HCMV, Allowing Comparative Analyses of Different Human Herpesviruses in One Reporter Cell Line.PLoS One. 2017 Jan 6;12(1):e0169580. doi: 10.1371/journal.pone.0169580. eCollection 2017. PLoS One. 2017. PMID: 28060895 Free PMC article.
-
Target highlights in CASP14: Analysis of models by structure providers.Proteins. 2021 Dec;89(12):1647-1672. doi: 10.1002/prot.26247. Epub 2021 Oct 10. Proteins. 2021. PMID: 34561912 Free PMC article.
References
-
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253–276. - PubMed
-
- Ludwig A, Hengel H. Epidemiological impact and disease burden of congenital cytomegalovirus infection in Europe. Euro Surveill. 2009;14:26–32. - PubMed
-
- Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93–99. - PubMed
-
- Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, et al. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol. 2000;11:283–290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

