The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1
- PMID: 21849636
- PMCID: PMC3213943
- DOI: 10.1152/ajpregu.00356.2011
The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1
Abstract
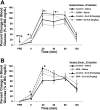
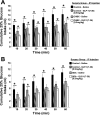
The incretin and food intake suppressive effects of intraperitoneally administered glucagon-like peptide-1 (GLP-1) involve activation of GLP-1 receptors (GLP-1R) expressed on vagal afferent fiber terminals. Central nervous system processing of GLP-1R-driven vagal afferents results in satiation signaling and enhanced insulin secretion from pancreatic-projecting vagal efferents. As the vast majority of endogenous GLP-1 is released from intestinal l-cells following ingestion, it stands to reason that paracrine GLP-1 signaling, activating adjacent GLP-1R expressed on vagal afferent fibers of gastrointestinal origin, contributes to glycemic and food intake control. However, systemic GLP-1R-mediated control of glycemia is currently attributed to endocrine action involving GLP-1R expressed in the hepatoportal bed on terminals of the common hepatic branch of the vagus (CHB). Here, we examine the hypothesis that activation of GLP-1R expressed on the CHB is not required for GLP-1's glycemic and intake suppressive effects, but rather paracrine signaling on non-CHB vagal afferents is required to mediate GLP-1's effects. Selective CHB ablation (CHBX), complete subdiaphragmatic vagal deafferentation (SDA), and surgical control rats received an oral glucose tolerance test (2.0 g glucose/kg) 10 min after an intraperitoneal injection of the GLP-1R antagonist, exendin-(9-39) (Ex-9; 0.5 mg/kg) or vehicle. CHBX and control rats showed comparable increases in blood glucose following blockade of GLP-1R by Ex-9, whereas SDA rats failed to show a GLP-1R-mediated incretin response. Furthermore, GLP-1(7-36) (0.5 mg/kg ip) produced a comparable suppression of 1-h 25% glucose intake in both CHBX and control rats, whereas intake suppression in SDA rats was blunted. These findings support the hypothesis that systemic GLP-1R mediation of glycemic control and food intake suppression involves paracrine-like signaling on GLP-1R expressed on vagal afferent fibers of gastrointestinal origin but does not require the CHB.
Figures
Similar articles
-
Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4.Endocrinology. 2011 Aug;152(8):3103-12. doi: 10.1210/en.2011-0174. Epub 2011 Jun 21. Endocrinology. 2011. PMID: 21693680 Free PMC article.
-
Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling.Endocrinology. 2009 Jun;150(6):2654-9. doi: 10.1210/en.2008-1479. Epub 2009 Mar 5. Endocrinology. 2009. PMID: 19264875 Free PMC article.
-
Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat.Neurogastroenterol Motil. 2009 Sep;21(9):978-e78. doi: 10.1111/j.1365-2982.2009.01317.x. Epub 2009 May 13. Neurogastroenterol Motil. 2009. PMID: 19453518
-
Vagal mediation of GLP-1's effects on food intake and glycemia.Physiol Behav. 2015 Dec 1;152(Pt B):372-80. doi: 10.1016/j.physbeh.2015.06.001. Epub 2015 Jun 3. Physiol Behav. 2015. PMID: 26048300 Review.
-
Neuronal and intracellular signaling pathways mediating GLP-1 energy balance and glycemic effects.Physiol Behav. 2012 Jun 6;106(3):413-6. doi: 10.1016/j.physbeh.2012.02.017. Epub 2012 Feb 17. Physiol Behav. 2012. PMID: 22366059 Free PMC article. Review.
Cited by
-
Oligofructose improves small intestinal lipid-sensing mechanisms via alterations to the small intestinal microbiota.Microbiome. 2023 Aug 2;11(1):169. doi: 10.1186/s40168-023-01590-2. Microbiome. 2023. PMID: 37533066 Free PMC article.
-
Mercaptoacetate blocks fatty acid-induced GLP-1 secretion in male rats by directly antagonizing GPR40 fatty acid receptors.Am J Physiol Regul Integr Comp Physiol. 2016 Apr 15;310(8):R724-32. doi: 10.1152/ajpregu.00387.2015. Epub 2016 Jan 20. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26791830 Free PMC article.
-
GLP-1 and weight loss: unraveling the diverse neural circuitry.Am J Physiol Regul Integr Comp Physiol. 2016 May 15;310(10):R885-95. doi: 10.1152/ajpregu.00520.2015. Epub 2016 Mar 30. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 27030669 Free PMC article. Review.
-
Actions of glucagon-like peptide-1 receptor ligands in the gut.Br J Pharmacol. 2022 Feb;179(4):727-742. doi: 10.1111/bph.15611. Epub 2021 Aug 4. Br J Pharmacol. 2022. PMID: 34235727 Free PMC article. Review.
-
Roux-en-Y gastric bypass operation in rats.J Vis Exp. 2012 Jun 11;(64):e3940. doi: 10.3791/3940. J Vis Exp. 2012. PMID: 22710348 Free PMC article.
References
-
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044: 127–131, 2005 - PubMed
-
- Ackroff K, Touzani K, Peets TK, Sclafani A. Flavor preferences conditioned by intragastric fructose and glucose: differences in reinforcement potency. Physiol Behav 72: 691–703, 2001 - PubMed
-
- Ahren B. Sensory nerves contribute to insulin secretion by glucagon-like peptide-1 in mice. Am J Physiol Regul Integr Comp Physiol 286: R269–R272, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

