C/EBPβ mediates tumour-induced ubiquitin ligase atrogin1/MAFbx upregulation and muscle wasting
- PMID: 21847090
- PMCID: PMC3199382
- DOI: 10.1038/emboj.2011.292
C/EBPβ mediates tumour-induced ubiquitin ligase atrogin1/MAFbx upregulation and muscle wasting
Abstract
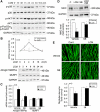
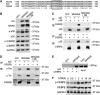
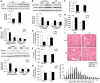
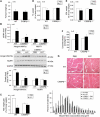
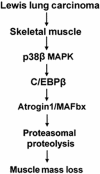
Upregulation of ubiquitin ligase atrogin1/MAFbx and muscle wasting are hallmarks of cancer cachexia; however, the underlying mechanism is undefined. Here, we describe a novel signalling pathway through which Lewis lung carcinoma (LLC) induces atrogin1/MAFbx upregulation and muscle wasting. C2C12 myotubes treated with LLC-conditioned medium (LCM) rapidly activates p38 MAPK and AKT while inactivating FoxO1/3, resulting in atrogin1/MAFbx upregulation, myosin heavy chain loss, and myotube atrophy. The p38α/β MAPK inhibitor SB202190 blocks the catabolic effects. Upon activation, p38 associates with C/EBPβ resulting in its phosphorylation and binding to a C/EBPβ-responsive cis-element in the atrogin1/MAFbx gene promoter. The promoter activity is stimulated by LCM via p38β-mediated activation of the C/EBPβ-responsive cis-element, independent of the adjacent FoxO1/3-responsive cis-elements in the promoter. In addition, p38 activation is observed in the muscle of LLC tumour-bearing mice, and SB202190 administration blocks atrogin1/MAFbx upregulation and muscle protein loss. Furthermore, C/EBPβ(-/-) mice are resistant to LLC tumour-induced atrogin1/MAFbx upregulation and muscle wasting. Therefore, activation of the p38β MAPK-C/EBPβ signalling pathway appears a key component of the pathogenesis of LLC tumour-induced cachexia.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
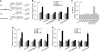
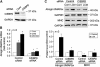
Similar articles
-
Valproic acid attenuates skeletal muscle wasting by inhibiting C/EBPβ-regulated atrogin1 expression in cancer cachexia.Am J Physiol Cell Physiol. 2016 Jul 1;311(1):C101-15. doi: 10.1152/ajpcell.00344.2015. Epub 2016 Apr 27. Am J Physiol Cell Physiol. 2016. PMID: 27122162
-
Signaling mechanism of tumor cell-induced up-regulation of E3 ubiquitin ligase UBR2.FASEB J. 2013 Jul;27(7):2893-901. doi: 10.1096/fj.12-222711. Epub 2013 Apr 8. FASEB J. 2013. PMID: 23568773 Free PMC article.
-
p38β MAPK upregulates atrogin1/MAFbx by specific phosphorylation of C/EBPβ.Skelet Muscle. 2012 Oct 9;2(1):20. doi: 10.1186/2044-5040-2-20. Skelet Muscle. 2012. PMID: 23046544 Free PMC article.
-
Signaling pathways perturbing muscle mass.Curr Opin Clin Nutr Metab Care. 2010 May;13(3):225-9. doi: 10.1097/mco.0b013e32833862df. Curr Opin Clin Nutr Metab Care. 2010. PMID: 20397318 Review.
-
Skeletal muscle hypertrophy and atrophy signaling pathways.Int J Biochem Cell Biol. 2005 Oct;37(10):1974-84. doi: 10.1016/j.biocel.2005.04.018. Int J Biochem Cell Biol. 2005. PMID: 16087388 Review.
Cited by
-
Atrogin1-induced loss of aquaporin 4 in myocytes leads to skeletal muscle atrophy.Sci Rep. 2020 Aug 25;10(1):14189. doi: 10.1038/s41598-020-71167-8. Sci Rep. 2020. PMID: 32843684 Free PMC article.
-
Edward F. Adolph Distinguished Lecture. Skeletal muscle atrophy: Multiple pathways leading to a common outcome.J Appl Physiol (1985). 2020 Aug 1;129(2):272-282. doi: 10.1152/japplphysiol.00381.2020. Epub 2020 Jul 9. J Appl Physiol (1985). 2020. PMID: 32644910 Free PMC article. Review.
-
Human pancreatic tumour organoid-derived factors enhance myogenic differentiation.J Cachexia Sarcopenia Muscle. 2022 Apr;13(2):1302-1313. doi: 10.1002/jcsm.12917. Epub 2022 Feb 11. J Cachexia Sarcopenia Muscle. 2022. PMID: 35146962 Free PMC article.
-
Cytokines and Chemokines in Cancer Cachexia and Its Long-Term Impact on COVID-19.Cells. 2022 Feb 8;11(3):579. doi: 10.3390/cells11030579. Cells. 2022. PMID: 35159388 Free PMC article. Review.
-
Skeletal muscle glycoprotein 130's role in Lewis lung carcinoma-induced cachexia.FASEB J. 2014 Feb;28(2):998-1009. doi: 10.1096/fj.13-240580. Epub 2013 Oct 21. FASEB J. 2014. PMID: 24145720 Free PMC article.
References
-
- Acharyya S, Butchbach ME, Sahenk Z, Wang H, Saji M, Carathers M, Ringel MD, Skipworth RJ, Fearon KC, Hollingsworth MA, Muscarella P, Burghes AH, Rafael-Fortney JA, Guttridge DC (2005) Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell 8: 421–432 - PubMed
-
- Acharyya S, Guttridge DC (2007) Cancer cachexia signaling pathways continue to emerge yet much still points to the proteasome. Clin Cancer Res 13: 1356–1361 - PubMed
-
- Andreyev HJ, Norman AR, Oates J, Cunningham D (1998) Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 34: 503–509 - PubMed
-
- Askari N, Beenstock J, Livnah O, Engelberg D (2009) p38 is active in vitro and in vivo when monophosphorylated on Thr180. Biochemistry 48: 2497–2504 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

