TLR7/9 versus TLR3/MDA5 signaling during virus infections and diabetes
- PMID: 21844166
- PMCID: PMC3177694
- DOI: 10.1189/jlb.0311166
TLR7/9 versus TLR3/MDA5 signaling during virus infections and diabetes
Abstract
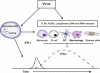
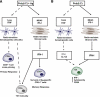
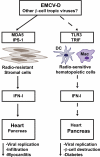
IFN-I are pleiotropic cytokines that impact innate and adaptive immune responses. In this article, we discuss TLR7/9 versus TLR3/MDA5 signaling in antiviral responses and diabetes. pDCs are thought to have a critical role in antiviral defense because of their ability to rapidly secrete large amounts of IFN-I through TLR7/9 signaling. A recent study demonstrates that although pDCs are a source of IFN-I in vivo, their overall contribution to viral containment is limited and time-dependent, such that additional cellular sources of IFN-I are required to fully control viral infections. dsRNA sensors, such as TLR3 and MDA5, provide another important trigger for antiviral IFN-I responses, which can be exploited to enhance immune responses to vaccines. In the absence of infection, IFN-I production by pDCs or from signaling through dsRNA sensors has been implicated in the pathogenesis of autoimmune diseases such as diabetes. However, recent data demonstrate that IFN-I production via TLR3 and MDA5 is critical to counter diabetes caused by a virus with preferential tropism for pancreatic β-cells. This highlights the complexity of the host antiviral response and how multiple cellular and molecular components balance protective versus pathological responses.
Figures
Similar articles
-
RNA sensor-induced type I IFN prevents diabetes caused by a β cell-tropic virus in mice.J Clin Invest. 2011 Apr;121(4):1497-507. doi: 10.1172/JCI44005. Epub 2011 Mar 14. J Clin Invest. 2011. PMID: 21403398 Free PMC article.
-
A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity.Proc Natl Acad Sci U S A. 2008 Dec 23;105(51):20446-51. doi: 10.1073/pnas.0810372105. Epub 2008 Dec 11. Proc Natl Acad Sci U S A. 2008. PMID: 19074283 Free PMC article.
-
Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses.J Immunol. 2009 Dec 1;183(11):6989-97. doi: 10.4049/jimmunol.0901386. Epub 2009 Nov 4. J Immunol. 2009. PMID: 19890046 Free PMC article.
-
Regulation of antiviral innate immune responses by RIG-I family of RNA helicases.Curr Top Microbiol Immunol. 2007;316:193-205. doi: 10.1007/978-3-540-71329-6_10. Curr Top Microbiol Immunol. 2007. PMID: 17969449 Review.
-
LGP2 synergy with MDA5 in RLR-mediated RNA recognition and antiviral signaling.Cytokine. 2015 Aug;74(2):198-206. doi: 10.1016/j.cyto.2015.02.010. Epub 2015 Mar 18. Cytokine. 2015. PMID: 25794939 Free PMC article. Review.
Cited by
-
IL-4 suppresses the responses to TLR7 and TLR9 stimulation and increases the permissiveness to retroviral infection of murine conventional dendritic cells.PLoS One. 2014 Jan 29;9(1):e87668. doi: 10.1371/journal.pone.0087668. eCollection 2014. PLoS One. 2014. PMID: 24489947 Free PMC article.
-
Autoimmune Regulator Expression in DC2.4 Cells Regulates the NF-κB Signaling and Cytokine Expression of the Toll-Like Receptor 3 Pathway.Int J Mol Sci. 2016 Dec 1;17(12):2002. doi: 10.3390/ijms17122002. Int J Mol Sci. 2016. PMID: 27916941 Free PMC article.
-
Immunological loss-of-function due to genetic gain-of-function in humans: autosomal dominance of the third kind.Curr Opin Immunol. 2015 Feb;32:90-105. doi: 10.1016/j.coi.2015.01.005. Epub 2015 Jan 31. Curr Opin Immunol. 2015. PMID: 25645939 Free PMC article. Review.
-
Poly (I:C)-Potentiated Vaccination Enhances T Cell Response in Olive Flounder (Paralichthys olivaceus) Providing Protection against Viral Hemorrhagic Septicemia Virus (VHSV).Vaccines (Basel). 2021 May 10;9(5):482. doi: 10.3390/vaccines9050482. Vaccines (Basel). 2021. PMID: 34068522 Free PMC article.
-
Natural killer cell activation contributes to hepatitis B viral control in a mouse model.Sci Rep. 2017 Mar 22;7(1):314. doi: 10.1038/s41598-017-00387-2. Sci Rep. 2017. PMID: 28331190 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

