Dendritic degeneration, neurovascular defects, and inflammation precede neuronal loss in a mouse model for tau-mediated neurodegeneration
- PMID: 21839061
- PMCID: PMC3181369
- DOI: 10.1016/j.ajpath.2011.06.025
Dendritic degeneration, neurovascular defects, and inflammation precede neuronal loss in a mouse model for tau-mediated neurodegeneration
Abstract
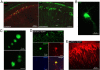
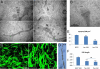
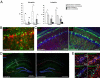
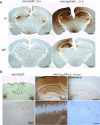
Adeno-associated virus (AAV)-mediated expression of wild-type or mutant P301L protein tau produces massive degeneration of pyramidal neurons without protein tau aggregation. We probed this novel model for genetic and structural factors and early parameters of pyramidal neurodegeneration. In yellow fluorescent protein-expressing transgenic mice, intracerebral injection of AAV-tauP301L revealed early damage to apical dendrites of CA1 pyramidal neurons, whereas their somata remained normal. Ultrastructurally, more and enlarged autophagic vacuoles were contained in degenerating dendrites and manifested as dark, discontinuous, vacuolated processes surrounded by activated astrocytes. Dendritic spines were lost in AAV-tauP301L-injected yellow fluorescent protein-expressing transgenic mice, and ultrastructurally, spines appeared dark and degenerating. In CX3CR1(EGFP/EGFP)-deficient mice, microglia were recruited early to neurons expressing human tau. The inflammatory response was accompanied by extravasation of plasma immunoglobulins. α2-Macroglobulin, but neither albumin nor transferrin, became lodged in the brain parenchyma. Large proteins, but not Evans blue, entered the brain of mice injected with AAV-tauP301L. Ultrastructurally, brain capillaries were constricted and surrounded by swollen astrocytes with extensions that contacted degenerating dendrites and axons. Together, these data corroborate the hypothesis that neuroinflammation participates essentially in tau-mediated neurodegeneration, and the model recapitulates early dendritic defects reminiscent of "dendritic amputation" in Alzheimer's disease.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Region-specific dendritic simplification induced by Aβ, mediated by tau via dysregulation of microtubule dynamics: a mechanistic distinct event from other neurodegenerative processes.Mol Neurodegener. 2015 Nov 5;10:60. doi: 10.1186/s13024-015-0049-0. Mol Neurodegener. 2015. PMID: 26541821 Free PMC article.
-
Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration.J Neurosci. 2005 Apr 6;25(14):3539-50. doi: 10.1523/JNEUROSCI.0081-05.2005. J Neurosci. 2005. PMID: 15814784 Free PMC article.
-
Ultrastructural neuronal pathology in transgenic mice expressing mutant (P301L) human tau.J Neurocytol. 2003 Nov;32(9):1091-105. doi: 10.1023/B:NEUR.0000021904.61387.95. J Neurocytol. 2003. PMID: 15044841
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
-
Transgenic mouse models of Alzheimer's disease.Ann N Y Acad Sci. 2000 Jun;908:260-6. doi: 10.1111/j.1749-6632.2000.tb06653.x. Ann N Y Acad Sci. 2000. PMID: 10911965 Review.
Cited by
-
Dysfunctional Sensory Modalities, Locus Coeruleus, and Basal Forebrain: Early Determinants that Promote Neuropathogenesis of Cognitive and Memory Decline and Alzheimer's Disease.Neurotox Res. 2016 Oct;30(3):295-337. doi: 10.1007/s12640-016-9643-3. Epub 2016 Jun 23. Neurotox Res. 2016. PMID: 27339162 Review.
-
Deficiency of terminal complement pathway inhibitor promotes neuronal tau pathology and degeneration in mice.J Neuroinflammation. 2012 Sep 18;9:220. doi: 10.1186/1742-2094-9-220. J Neuroinflammation. 2012. PMID: 22989354 Free PMC article.
-
The polarity protein partitioning-defective 1 (PAR-1) regulates dendritic spine morphogenesis through phosphorylating postsynaptic density protein 95 (PSD-95).J Biol Chem. 2012 Aug 31;287(36):30781-8. doi: 10.1074/jbc.M112.351452. Epub 2012 Jul 17. J Biol Chem. 2012. PMID: 22807451 Free PMC article.
-
Publication Trends for Alzheimer's Disease Worldwide and in China: A 30-Year Bibliometric Analysis.Front Hum Neurosci. 2019 Aug 9;13:259. doi: 10.3389/fnhum.2019.00259. eCollection 2019. Front Hum Neurosci. 2019. PMID: 31447661 Free PMC article.
-
Phospholipase D1 Attenuation Therapeutics Promotes Resilience against Synaptotoxicity in 12-Month-Old 3xTg-AD Mouse Model of Progressive Neurodegeneration.Int J Mol Sci. 2023 Feb 8;24(4):3372. doi: 10.3390/ijms24043372. Int J Mol Sci. 2023. PMID: 36834781 Free PMC article.
References
-
- Jaworski T., Dewachter I., Lechat B., Croes S., Termont A., Demedts D., Borghgraef P., Devijver H., Filipkowski R.K., Kaczmarek L., Kügler S., Van Leuven F. AAV-tau mediates pyramidal neurodegeneration by cell-cycle re-entry without neurofibrillary tangle formation in WT mice. PLoS One. 2009;4:e7280. - PMC - PubMed
-
- Braak E., Braak H. Alzheimer's disease: transiently developing dendritic changes in pyramidal cells of sector CA1 of the Ammon's horn. Acta Neuropathol. 1997;93:323–325. - PubMed
-
- Jaworski T., Dewachter I., Seymour C.M., Borghgraef P., Devijver H., Kügler S., Van Leuven F. Alzheimer's disease: old problem, new views from transgenic and viral models. Biochim Biophys Acta. 2010;1802:808–818. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

