Supernatants from lymphocytes stimulated with Bacillus Calmette-Guerin can modify the antigenicity of tumours and stimulate allogeneic T-cell responses
- PMID: 21829193
- PMCID: PMC3188926
- DOI: 10.1038/bjc.2011.306
Supernatants from lymphocytes stimulated with Bacillus Calmette-Guerin can modify the antigenicity of tumours and stimulate allogeneic T-cell responses
Abstract
Background: Reduced expression of class 1 human leucocyte antigens (HLA1) is often a mechanism by which tumours evade surveillance by the host immune system. This is often associated with an immune function that is unable to mount appropriate responses against disease, which can result in a state that favours carcinogenesis.
Methods: In the current study, we have explored the effects of Bacillus Calmette-Guerin (BCG) on the cytokine output of leucocytes, which is a key determinant in generating antitumour action, and have also assessed the effect of these cytokine cocktails on HLA1 expression in solid tumour cell lines.
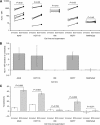
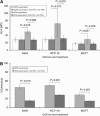
Results: BCG potently activated a broad range of leucocytes, and also enhanced the production of cytokines that were Th(1)-predominant. Supernatants from BCG-treated leucocytes significantly increased the expression of HLA1 on the surface of cancer cell lines, which correlated with increased cytolytic T-cell activity. We also showed that the increased HLA1 expression was associated with activation of intracellular signalling pathways, which was triggered by the increases in the Th(1)-cytokines interferon-γ and tumour necrosis factor-α, as counteracting their effects negated the enhancement.
Conclusion: These studies reaffirm the role of BCG as a putative immunotherapy through their cytokine-modifying effects on leucocytes and their capacity to enhance tumour visibility.
Figures



Similar articles
-
Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses.Br J Cancer. 2010 Jan 5;102(1):115-23. doi: 10.1038/sj.bjc.6605465. Epub 2009 Dec 8. Br J Cancer. 2010. PMID: 19997099 Free PMC article.
-
Induction of bacillus-Calmette-Guérin-activated killer cells from human peripheral blood mononuclear cells against human bladder carcinoma cell lines in vitro.Cancer Immunol Immunother. 1993 Jul;37(2):105-11. doi: 10.1007/BF01517042. Cancer Immunol Immunother. 1993. PMID: 8319241 Free PMC article.
-
Recombinant bacillus Calmette-Guérin (BCG) expressing interferon-alpha 2B enhances human mononuclear cell cytotoxicity against bladder cancer cell lines in vitro.Cancer Immunol Immunother. 2009 Oct;58(10):1647-55. doi: 10.1007/s00262-009-0673-z. Epub 2009 Feb 13. Cancer Immunol Immunother. 2009. PMID: 19214503 Free PMC article.
-
Role of urothelial cells in BCG immunotherapy for superficial bladder cancer.Br J Cancer. 2004 Aug 16;91(4):607-12. doi: 10.1038/sj.bjc.6602026. Br J Cancer. 2004. PMID: 15266312 Free PMC article. Review.
-
Immune response following intravesical bacillus Calmette-Guerin instillations in superficial bladder cancer: a review.Urol Res. 1998;26(3):155-9. doi: 10.1007/s002400050039. Urol Res. 1998. PMID: 9694595 Review.
Cited by
-
Repurposing Infectious Diseases Vaccines Against Cancer.Front Oncol. 2021 May 13;11:688755. doi: 10.3389/fonc.2021.688755. eCollection 2021. Front Oncol. 2021. PMID: 34055652 Free PMC article. Review.
-
Supernatants derived from chemotherapy-treated cancer cell lines can modify angiogenesis.Br J Cancer. 2012 Feb 28;106(5):896-903. doi: 10.1038/bjc.2012.13. Epub 2012 Jan 31. Br J Cancer. 2012. PMID: 22294186 Free PMC article.
References
-
- Abbas AK, Murphy KM, Sher A (1996) Functional diversity of helper T lymphocytes. Nature 383: 787–793 - PubMed
-
- Alexandroff AB, Jackson AM, O’Donnell MA, James K (1999) BCG immunotherapy of bladder cancer: 20 years on. Lancet 353: 1689–1694 - PubMed
-
- Aptsiauri N, Cabrera T, Mendez R, Garcia-Lora A, Ruiz-Cabello F, Garrido F (2007) Role of altered expression of HLA class I molecules in cancer progression. Adv Exp Med Biol 601: 123–131 - PubMed
-
- Bach EA, Aguet M, Schreiber RD (1997) The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol 15: 563–591 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

