Cool-1/βPIX functions as a guanine nucleotide exchange factor in the cycling of Cdc42 to regulate insulin secretion
- PMID: 21828338
- PMCID: PMC3233779
- DOI: 10.1152/ajpendo.00312.2011
Cool-1/βPIX functions as a guanine nucleotide exchange factor in the cycling of Cdc42 to regulate insulin secretion
Abstract
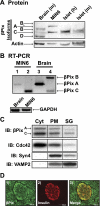
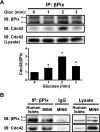
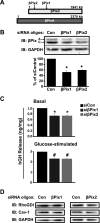
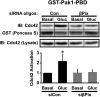
Second-phase insulin release requires the sustained mobilization of insulin granules from internal storage pools to the cell surface for fusion with the plasma membrane. However, the detailed mechanisms underlying this process remain largely unknown. GTP-loading of the small GTPase Cdc42 is the first glucose-specific activation step in the process, although how glucose triggers Cdc42 activation is entirely unknown. In a directed candidate screen for guanine nucleotide exchange factors (GEFs), which directly activate small GTPases, Cool-1/βPix was identified in pancreatic islet beta cells. In support of its role as the beta cell Cdc42 GEF, βPix coimmunoprecipitated with Cdc42 in human islets and MIN6 beta cells in a glucose-dependent manner, peaking just prior to Cdc42 activation. Furthermore, RNAi-mediated βPix reduction by 50% corresponded to full ablation of glucose-induced Cdc42 activation and significant attenuation of basal and glucose-stimulated insulin secretion. Of the two Cdc42 guanine nucleotide dissociation inhibitor (GDI) proteins identified in beta cells, βPix competed selectively with caveolin-1 (Cav-1) but not RhoGDI in coimmunoprecipitation and GST-Cdc42-GDP interaction assays. However, a phospho-deficient Cav-1-Y14F mutant failed to compete with βPix; Cav-1(Tyr14) is an established phosphorylation site for Src kinase. Taken together, these data support a new model, wherein glucose stimulates Cav-1 and induces its dissociation from Cdc42, possibly via Src kinase activation to phosphorylate Cav-1(Tyr14), to promote Cdc42-βPix binding and Cdc42 activation, and to trigger downstream signaling and ultimately sustain insulin release.
Figures
Comment in
-
Second-phase insulin secretion gets cool.Am J Physiol Endocrinol Metab. 2011 Dec;301(6):E1070-1. doi: 10.1152/ajpendo.00491.2011. Epub 2011 Sep 20. Am J Physiol Endocrinol Metab. 2011. PMID: 21934039 No abstract available.
Similar articles
-
Emerging Roles of Small GTPases in Islet β-Cell Function.Cells. 2021 Jun 15;10(6):1503. doi: 10.3390/cells10061503. Cells. 2021. PMID: 34203728 Free PMC article. Review.
-
Caveolin-1 functions as a novel Cdc42 guanine nucleotide dissociation inhibitor in pancreatic beta-cells.J Biol Chem. 2006 Jul 14;281(28):18961-72. doi: 10.1074/jbc.M603604200. Epub 2006 May 19. J Biol Chem. 2006. PMID: 16714282
-
Differential phosphorylation of RhoGDI mediates the distinct cycling of Cdc42 and Rac1 to regulate second-phase insulin secretion.J Biol Chem. 2010 Feb 26;285(9):6186-97. doi: 10.1074/jbc.M109.072421. Epub 2009 Dec 22. J Biol Chem. 2010. PMID: 20028975 Free PMC article.
-
YES, a Src family kinase, is a proximal glucose-specific activator of cell division cycle control protein 42 (Cdc42) in pancreatic islet β cells.J Biol Chem. 2014 Apr 18;289(16):11476-11487. doi: 10.1074/jbc.M114.559328. Epub 2014 Mar 7. J Biol Chem. 2014. PMID: 24610809 Free PMC article.
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
Cited by
-
Loss of Caveolin-1 Is Associated with a Decrease in Beta Cell Death in Mice on a High Fat Diet.Int J Mol Sci. 2020 Jul 23;21(15):5225. doi: 10.3390/ijms21155225. Int J Mol Sci. 2020. PMID: 32718046 Free PMC article.
-
Integrated analysis of mRNAs and lncRNAs reveals candidate marker genes and potential hub lncRNAs associated with growth regulation of the Pacific Oyster, Crassostrea gigas.BMC Genomics. 2023 Aug 10;24(1):453. doi: 10.1186/s12864-023-09543-7. BMC Genomics. 2023. PMID: 37563567 Free PMC article.
-
Emerging Roles of Small GTPases in Islet β-Cell Function.Cells. 2021 Jun 15;10(6):1503. doi: 10.3390/cells10061503. Cells. 2021. PMID: 34203728 Free PMC article. Review.
-
Serotonin Influences Insulin Secretion in Rat Insulinoma INS-1E Cells.Int J Mol Sci. 2024 Jun 21;25(13):6828. doi: 10.3390/ijms25136828. Int J Mol Sci. 2024. PMID: 38999937 Free PMC article.
-
Hyperglycemic Stress Induces Expression, Degradation, and Nuclear Association of Rho GDP Dissociation Inhibitor 2 (RhoGDIβ) in Pancreatic β-Cells.Cells. 2024 Feb 1;13(3):272. doi: 10.3390/cells13030272. Cells. 2024. PMID: 38334664 Free PMC article.
References
-
- Audebert S, Navarro C, Nourry C, Chasserot-Golaz S, Lecine P, Bellaiche Y, Dupont JL, Premont RT, Sempere C, Strub JM, Van Dorsselaer A, Vitale N, Borg JP. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr Biol 14: 987–995, 2004 - PubMed
-
- Barber MA, Donald S, Thelen S, Anderson KE, Thelen M, Welch HC. Membrane translocation of P-Rex1 is mediated by G protein betagamma subunits and phosphoinositide 3-kinase. J Biol Chem 282: 29967–29976, 2007 - PubMed
-
- Bokoch GM. Biology of the P21-activated kinases. Annu Rev Biochem 72: 743–781, 2003 - PubMed
-
- Cheng H, Straub SG, Sharp GW. Inhibitory role of Src family tyrosine kinases on Ca2+-dependent insulin release. Am J Physiol Endocrinol Metab 292: E845–E852, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

