The aneurogenic limb identifies developmental cell interactions underlying vertebrate limb regeneration
- PMID: 21825124
- PMCID: PMC3158191
- DOI: 10.1073/pnas.1108472108
The aneurogenic limb identifies developmental cell interactions underlying vertebrate limb regeneration
Abstract
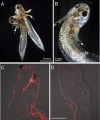
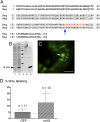
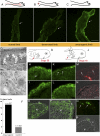
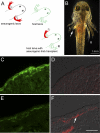
The removal of the neural tube in salamander embryos allows the development of nerve-free aneurogenic limbs. Limb regeneration is normally nerve-dependent, but the aneurogenic limb regenerates without nerves and becomes nerve-dependent after innervation. The molecular basis for these tissue interactions is unclear. Anterior Gradient (AG) protein, previously shown to rescue regeneration of denervated limbs and to act as a growth factor for cultured limb blastemal cells, is expressed throughout the larval limb epidermis and is down-regulated by innervation. In an aneurogenic limb, the level of AG protein remains high in the epidermis throughout development and regeneration, but decreases after innervation following transplantation to a normal host. Aneurogenic epidermis also shows a fivefold difference in secretory gland cells, which express AG protein. The persistently high expression of AG in the epithelial cells of an aneurogenic limb ensures that regeneration is independent of the nerve. These findings provide an explanation for this classical problem, and identify regulation of the epidermal niche by innervation as a distinctive developmental mechanism that initiates the nerve dependence of limb regeneration. The absence of this regulation during anuran limb development might suggest that it evolved in relation to limb regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Generation of aneurogenic larvae by parabiosis of salamander embryos.Methods Mol Biol. 2015;1290:147-57. doi: 10.1007/978-1-4939-2495-0_12. Methods Mol Biol. 2015. PMID: 25740484
-
The nerve dependence of amphibian limb regeneration.J Exp Biol. 1987 Sep;132:79-91. doi: 10.1242/jeb.132.1.79. J Exp Biol. 1987. PMID: 3323408 Review.
-
Responses to amputation of denervated ambystoma limbs containing aneurogenic limb grafts.J Exp Zool A Comp Exp Biol. 2003 May 1;297(1):64-79. doi: 10.1002/jez.a.10263. J Exp Zool A Comp Exp Biol. 2003. PMID: 12911114
-
Nerve dependence in tissue, organ, and appendage regeneration.Trends Neurosci. 2012 Nov;35(11):691-9. doi: 10.1016/j.tins.2012.08.003. Epub 2012 Sep 16. Trends Neurosci. 2012. PMID: 22989534 Review.
-
A comparative study of gland cells implicated in the nerve dependence of salamander limb regeneration.J Anat. 2010 Jul;217(1):16-25. doi: 10.1111/j.1469-7580.2010.01239.x. Epub 2010 Apr 26. J Anat. 2010. PMID: 20456522 Free PMC article.
Cited by
-
AGR2 gene function requires a unique endoplasmic reticulum localization motif.J Biol Chem. 2012 Feb 10;287(7):4773-82. doi: 10.1074/jbc.M111.301531. Epub 2011 Dec 19. J Biol Chem. 2012. PMID: 22184114 Free PMC article.
-
The salamander blastema within the broader context of metazoan regeneration.Front Cell Dev Biol. 2023 Aug 11;11:1206157. doi: 10.3389/fcell.2023.1206157. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37635872 Free PMC article. Review.
-
A brief history of the study of nerve dependent regeneration.Neurogenesis (Austin). 2017 Apr 10;4(1):e1302216. doi: 10.1080/23262133.2017.1302216. eCollection 2017. Neurogenesis (Austin). 2017. PMID: 28459075 Free PMC article. Review.
-
Salamanders: The molecular basis of tissue regeneration and its relevance to human disease.Curr Top Dev Biol. 2021;145:235-275. doi: 10.1016/bs.ctdb.2020.11.009. Epub 2021 Mar 16. Curr Top Dev Biol. 2021. PMID: 34074531 Free PMC article. Review.
-
Neural regeneration: lessons from regenerating and non-regenerating systems.Mol Neurobiol. 2012 Oct;46(2):227-41. doi: 10.1007/s12035-012-8290-9. Epub 2012 Jun 21. Mol Neurobiol. 2012. PMID: 22717989 Review.
References
-
- Hall SM. Mechanisms of repair after traumatic injury. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. 4th Ed. Philadelphia: Elsevier; 2005. pp. 1403–1434.
-
- Singer M. The influence of the nerve in regeneration of the amphibian extremity. Q Rev Biol. 1952;27:169–200. - PubMed
-
- Goldhamer DJ, Tomlinson BL, Tassava RA. Ganglia implantation as a means of supplying neurotrophic stimulation to the newt regeneration blastema: Cell-cycle effects in innervated and denervated limbs. J Exp Zool. 1992;262:71–80. - PubMed
-
- Kintner CR, Brockes JP. Monoclonal antibodies to the cells of a regenerating limb. J Embryol Exp Morphol. 1985;89:37–55. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

