Development of a surface plasmon resonance biosensor for real-time detection of osteogenic differentiation in live mesenchymal stem cells
- PMID: 21818317
- PMCID: PMC3144898
- DOI: 10.1371/journal.pone.0022382
Development of a surface plasmon resonance biosensor for real-time detection of osteogenic differentiation in live mesenchymal stem cells
Abstract
Surface plasmon resonance (SPR) biosensors have been recognized as a useful tool and widely used for real-time dynamic analysis of molecular binding affinity because of its high sensitivity to the change of the refractive index of tested objects. The conventional methods in molecular biology to evaluate cell differentiation require cell lysis or fixation, which make investigation in live cells difficult. In addition, a certain amount of cells are needed in order to obtain adequate protein or messenger ribonucleic acid for various assays. To overcome this limitation, we developed a unique SPR-based biosensing apparatus for real-time detection of cell differentiation in live cells according to the differences of optical properties of the cell surface caused by specific antigen-antibody binding. In this study, we reported the application of this SPR-based system to evaluate the osteogenic differentiation of mesenchymal stem cells (MSCs). OB-cadherin expression, which is up-regulated during osteogenic differentiation, was targeted under our SPR system by conjugating antibodies against OB-cadherin on the surface of the object. A linear relationship between the duration of osteogenic induction and the difference in refractive angle shift with very high correlation coefficient was observed. To sum up, the SPR system and the protocol reported in this study can rapidly and accurately define osteogenic maturation of MSCs in a live cell and label-free manner with no need of cell breakage. This SPR biosensor will facilitate future advances in a vast array of fields in biomedical research and medical diagnosis.
Conflict of interest statement
Figures
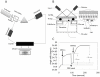


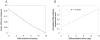
 , as a function of culture time. (B) OB-cadherin expression normalized to SaOS2 during differentiation.
, as a function of culture time. (B) OB-cadherin expression normalized to SaOS2 during differentiation.Similar articles
-
Early-stage detection of VE-cadherin during endothelial differentiation of human mesenchymal stem cells using SPR biosensor.Biosens Bioelectron. 2017 Oct 15;96:358-366. doi: 10.1016/j.bios.2017.05.018. Epub 2017 May 10. Biosens Bioelectron. 2017. PMID: 28527412
-
Preliminary approach of real-time monitoring in vitro matrix mineralization based on surface plasmon resonance detection.Biotechnol Bioeng. 2011 Jun;108(6):1473-8. doi: 10.1002/bit.23049. Epub 2011 Jan 17. Biotechnol Bioeng. 2011. PMID: 21192003
-
Detection of osteogenic differentiation by differential mineralized matrix production in mesenchymal stromal cells by Raman spectroscopy.PLoS One. 2013 May 29;8(5):e65438. doi: 10.1371/journal.pone.0065438. Print 2013. PLoS One. 2013. PMID: 23734254 Free PMC article.
-
Rapid and label-free bacteria detection by surface plasmon resonance (SPR) biosensors.Biotechnol J. 2009 Jul;4(7):1003-11. doi: 10.1002/biot.200800316. Biotechnol J. 2009. PMID: 19288516 Review.
-
Surface plasmon resonance based biosensor technique: a review.J Biophotonics. 2012 Jul;5(7):483-501. doi: 10.1002/jbio.201200015. Epub 2012 Mar 30. J Biophotonics. 2012. PMID: 22467335 Review.
Cited by
-
The Current Trends of Biosensors in Tissue Engineering.Biosensors (Basel). 2020 Aug 3;10(8):88. doi: 10.3390/bios10080088. Biosensors (Basel). 2020. PMID: 32756393 Free PMC article. Review.
-
Exploring the Association of Surface Plasmon Resonance with Recombinant MHC:Ig Hybrid Protein as a Tool for Detecting T Lymphocytes in Mice Infected with Leishmania (Leishmania) amazonensis.Biomed Res Int. 2017;2017:9089748. doi: 10.1155/2017/9089748. Epub 2017 Mar 8. Biomed Res Int. 2017. PMID: 28373990 Free PMC article.
-
Quick and label-free detection for Coumaphos by using surface plasmon resonance biochip.PLoS One. 2014 Aug 14;9(8):e104689. doi: 10.1371/journal.pone.0104689. eCollection 2014. PLoS One. 2014. PMID: 25122502 Free PMC article.
-
Quantification of mesenchymal stem cell growth rates through secretory and excretory biomolecules in conditioned media via Fresnel reflection.Sensors (Basel). 2013 Sep 30;13(10):13276-88. doi: 10.3390/s131013276. Sensors (Basel). 2013. PMID: 24084118 Free PMC article.
-
Biosensors in clinical practice: focus on oncohematology.Sensors (Basel). 2013 May 14;13(5):6423-47. doi: 10.3390/s130506423. Sensors (Basel). 2013. PMID: 23673681 Free PMC article. Review.
References
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Smith JR, Pochampally R, Perry A, Hsu SC, Prockop DJ. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells. 2004;22:823–831. - PubMed
-
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WWK, Gordon PL, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nature Medicine. 1999;5:309–313. - PubMed
-
- Pereira RF, Halford KW, Ohara MD, Leeper DB, Sokolov BP, et al. Cultured Adherent Cells from Marrow Can Serve as Long-Lasting Precursor Cells for Bone, Cartilage, and Lung in Irradiated Mice. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4857–4861. - PMC - PubMed
-
- Jiang YH, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

