SHH1, a homeodomain protein required for DNA methylation, as well as RDR2, RDM4, and chromatin remodeling factors, associate with RNA polymerase IV
- PMID: 21811420
- PMCID: PMC3141008
- DOI: 10.1371/journal.pgen.1002195
SHH1, a homeodomain protein required for DNA methylation, as well as RDR2, RDM4, and chromatin remodeling factors, associate with RNA polymerase IV
Abstract
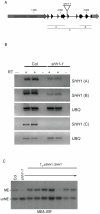
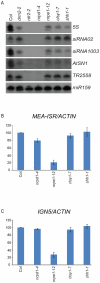
DNA methylation is an evolutionarily conserved epigenetic modification that is critical for gene silencing and the maintenance of genome integrity. In Arabidopsis thaliana, the de novo DNA methyltransferase, domains rearranged methyltransferase 2 (DRM2), is targeted to specific genomic loci by 24 nt small interfering RNAs (siRNAs) through a pathway termed RNA-directed DNA methylation (RdDM). Biogenesis of the targeting siRNAs is thought to be initiated by the activity of the plant-specific RNA polymerase IV (Pol-IV). However, the mechanism through which Pol-IV is targeted to specific genomic loci and whether factors other than the core Pol-IV machinery are required for Pol-IV activity remain unknown. Through the affinity purification of nuclear RNA polymerase D1 (NRPD1), the largest subunit of the Pol-IV polymerase, we found that several previously identified RdDM components co-purify with Pol-IV, namely RNA-dependent RNA polymerase 2 (RDR2), CLASSY1 (CLSY1), and RNA-directed DNA methylation 4 (RDM4), suggesting that the upstream siRNA generating portion of the RdDM pathway may be more physically coupled than previously envisioned. A homeodomain protein, SAWADEE homeodomain homolog 1 (SHH1), was also found to co-purify with NRPD1; and we demonstrate that SHH1 is required for de novo and maintenance DNA methylation, as well as for the accumulation of siRNAs at specific loci, confirming it is a bonafide component of the RdDM pathway.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Polymerase IV occupancy at RNA-directed DNA methylation sites requires SHH1.Nature. 2013 Jun 20;498(7454):385-9. doi: 10.1038/nature12178. Epub 2013 May 1. Nature. 2013. PMID: 23636332 Free PMC article.
-
DTF1 is a core component of RNA-directed DNA methylation and may assist in the recruitment of Pol IV.Proc Natl Acad Sci U S A. 2013 May 14;110(20):8290-5. doi: 10.1073/pnas.1300585110. Epub 2013 May 1. Proc Natl Acad Sci U S A. 2013. PMID: 23637343 Free PMC article.
-
The Pol IV largest subunit CTD quantitatively affects siRNA levels guiding RNA-directed DNA methylation.Nucleic Acids Res. 2019 Sep 26;47(17):9024-9036. doi: 10.1093/nar/gkz615. Nucleic Acids Res. 2019. PMID: 31329950 Free PMC article.
-
Advances of RNA polymerase IV in controlling DNA methylation and development in plants.Yi Chuan. 2022 Jul 20;44(7):567-580. doi: 10.16288/j.yczz.22-063. Yi Chuan. 2022. PMID: 35858769 Review.
-
RNA-directed DNA methylation in plants: Where to start?RNA Biol. 2013 Oct;10(10):1593-6. doi: 10.4161/rna.26312. RNA Biol. 2013. PMID: 25003825 Free PMC article. Review.
Cited by
-
The splicing machinery promotes RNA-directed DNA methylation and transcriptional silencing in Arabidopsis.EMBO J. 2013 Apr 17;32(8):1128-40. doi: 10.1038/emboj.2013.49. Epub 2013 Mar 22. EMBO J. 2013. PMID: 23524848 Free PMC article.
-
A SWI/SNF chromatin-remodeling complex acts in noncoding RNA-mediated transcriptional silencing.Mol Cell. 2013 Jan 24;49(2):298-309. doi: 10.1016/j.molcel.2012.11.011. Epub 2012 Dec 13. Mol Cell. 2013. PMID: 23246435 Free PMC article.
-
Unusual case of apparent hypermutation in Arabidopsis thaliana.Genetics. 2012 Dec;192(4):1271-80. doi: 10.1534/genetics.112.144634. Epub 2012 Sep 28. Genetics. 2012. PMID: 23023006 Free PMC article.
-
Establishment, maintenance, and biological roles of non-CG methylation in plants.Essays Biochem. 2019 Dec 20;63(6):743-755. doi: 10.1042/EBC20190032. Essays Biochem. 2019. PMID: 31652316 Free PMC article. Review.
-
Interplay between active chromatin marks and RNA-directed DNA methylation in Arabidopsis thaliana.PLoS Genet. 2013 Nov;9(11):e1003946. doi: 10.1371/journal.pgen.1003946. Epub 2013 Nov 7. PLoS Genet. 2013. PMID: 24244201 Free PMC article.
References
-
- Bostick M, Kim JK, Esteve PO, Clark A, Pradhan S, et al. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007;317:1760–1764. - PubMed
-
- Sharif J, Muto M, Takebayashi S, Suetake I, Iwamatsu A, et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature. 2007;450:908–912. - PubMed
-
- Matzke M, Kanno T, Daxinger L, Huettel B, Matzke AJ. RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol. 2009;21:367–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

