Receptors and tropisms of envelope viruses
- PMID: 21804908
- PMCID: PMC3144558
- DOI: 10.1016/j.coviro.2011.05.001
Receptors and tropisms of envelope viruses
Abstract
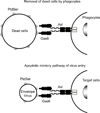
Envelope virus replication begins with receptor binding, followed by fusion of the viral envelope with the cell membrane. The binding and fusion steps are usually mediated by envelope proteins. The ability of envelope proteins of a particular virus to bind and fuse with target cells defines the host range of the virus, known as 'viral tropism'. The mechanism(s) of fusion by the viral envelope is largely categorized as either pH-dependent or pH-independent. By redirecting the binding specificities of envelope proteins to desired target molecules while maintaining fusion activity, it is possible to redirect the tropisms of virus and viral vectors, enabling specific killing and/or transduction of desired cells in vivo. Recently, a lipid, phosphatidylserine, was also shown to mediate binding of virus, which affects the tropisms of viruses and viral vectors.
Figures


Similar articles
-
Role of phosphatidylserine receptors in enveloped virus infection.J Virol. 2014 Apr;88(8):4275-90. doi: 10.1128/JVI.03287-13. Epub 2014 Jan 29. J Virol. 2014. PMID: 24478428 Free PMC article.
-
Cell entry of enveloped viruses.Curr Opin Virol. 2011 Aug;1(2):92-100. doi: 10.1016/j.coviro.2011.06.002. Curr Opin Virol. 2011. PMID: 21927634 Free PMC article. Review.
-
Characterizing functional domains for TIM-mediated enveloped virus entry.J Virol. 2014 Jun;88(12):6702-13. doi: 10.1128/JVI.00300-14. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696470 Free PMC article.
-
Cell entry of enveloped viruses.Adv Genet. 2011;73:121-83. doi: 10.1016/B978-0-12-380860-8.00004-5. Adv Genet. 2011. PMID: 21310296 Free PMC article. Review.
-
Host receptors: the key to establishing cells with broad viral tropism for vaccine production.Crit Rev Microbiol. 2020 Mar;46(2):147-168. doi: 10.1080/1040841X.2020.1735992. Epub 2020 Mar 23. Crit Rev Microbiol. 2020. PMID: 32202955 Free PMC article. Review.
Cited by
-
Structural characterization of a fusion glycoprotein from a retrovirus that undergoes a hybrid 2-step entry mechanism.FASEB J. 2013 Dec;27(12):5059-71. doi: 10.1096/fj.13-232371. Epub 2013 Sep 13. FASEB J. 2013. PMID: 24036886 Free PMC article.
-
Controlling AAV Tropism in the Nervous System with Natural and Engineered Capsids.Methods Mol Biol. 2016;1382:133-49. doi: 10.1007/978-1-4939-3271-9_10. Methods Mol Biol. 2016. PMID: 26611584 Free PMC article.
-
Bovine Nebovirus Interacts with a Wide Spectrum of Histo-Blood Group Antigens.J Virol. 2018 Apr 13;92(9):e02160-17. doi: 10.1128/JVI.02160-17. Print 2018 May 1. J Virol. 2018. PMID: 29467317 Free PMC article.
-
Versatile targeting system for lentiviral vectors involving biotinylated targeting molecules.Virology. 2018 Dec;525:170-181. doi: 10.1016/j.virol.2018.09.017. Epub 2018 Oct 2. Virology. 2018. PMID: 30290312 Free PMC article.
-
Retrovirus entry by endocytosis and cathepsin proteases.Adv Virol. 2012;2012:640894. doi: 10.1155/2012/640894. Epub 2012 Dec 6. Adv Virol. 2012. PMID: 23304142 Free PMC article.
References
-
- Norkin LC. Virology, Molecular biology and pathogenesis. ASM press; 2010.
-
- Fields BN, Knipe DM, Howley PM. Fields' virology. edn 5th. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007.
-
- Mothes W, Boerger AL, Narayan S, Cunningham JM, Young JA. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell. 2000;103:679–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

