Coordinated host responses during pyroptosis: caspase-1-dependent lysosome exocytosis and inflammatory cytokine maturation
- PMID: 21804020
- PMCID: PMC3660150
- DOI: 10.4049/jimmunol.1100477
Coordinated host responses during pyroptosis: caspase-1-dependent lysosome exocytosis and inflammatory cytokine maturation
Abstract
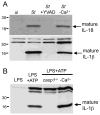
Activation of caspase-1 leads to pyroptosis, a program of cell death characterized by cell lysis and inflammatory cytokine release. Caspase-1 activation triggered by multiple nucleotide-binding oligomerization domain-like receptors (NLRs; NLRC4, NLRP1b, or NLRP3) leads to loss of lysosomes via their fusion with the cell surface, or lysosome exocytosis. Active caspase-1 increased cellular membrane permeability and intracellular calcium levels, which facilitated lysosome exocytosis and release of host antimicrobial factors and microbial products. Lysosome exocytosis has been proposed to mediate secretion of IL-1β and IL-18; however, blocking lysosome exocytosis did not alter cytokine processing or release. These studies indicate two conserved secretion pathways are initiated by caspase-1, lysosome exocytosis, and a parallel pathway resulting in cytokine release, and both enhance the antimicrobial nature of pyroptosis.
Figures

Similar articles
-
Monitoring Calcium Fluxes and Lysosome Exocytosis During Pyroptosis.Methods Mol Biol. 2023;2641:171-178. doi: 10.1007/978-1-0716-3040-2_14. Methods Mol Biol. 2023. PMID: 37074650
-
Asc modulates the function of NLRC4 in response to infection of macrophages by Legionella pneumophila.mBio. 2011 Sep 1;2(4):e00117-11. doi: 10.1128/mBio.00117-11. Print 2011. mBio. 2011. PMID: 21771913 Free PMC article.
-
Caspase-1 Engagement and TLR-Induced c-FLIP Expression Suppress ASC/Caspase-8-Dependent Apoptosis by Inflammasome Sensors NLRP1b and NLRC4.Cell Rep. 2017 Dec 19;21(12):3427-3444. doi: 10.1016/j.celrep.2017.11.088. Cell Rep. 2017. PMID: 29262324 Free PMC article.
-
P2X7 receptor regulation of non-classical secretion from immune effector cells.Cell Microbiol. 2012 Nov;14(11):1697-706. doi: 10.1111/cmi.12001. Epub 2012 Aug 24. Cell Microbiol. 2012. PMID: 22882764 Free PMC article. Review.
-
Caspase-11: the driving factor for noncanonical inflammasomes.Eur J Immunol. 2013 Sep;43(9):2240-5. doi: 10.1002/eji.201343800. Eur J Immunol. 2013. PMID: 24037676 Review.
Cited by
-
Burkholderia cenocepacia type VI secretion system mediates escape of type II secreted proteins into the cytoplasm of infected macrophages.PLoS One. 2012;7(7):e41726. doi: 10.1371/journal.pone.0041726. Epub 2012 Jul 25. PLoS One. 2012. PMID: 22848580 Free PMC article.
-
LncRNA-Fendrr protects against the ubiquitination and degradation of NLRC4 protein through HERC2 to regulate the pyroptosis of microglia.Mol Med. 2021 Apr 15;27(1):39. doi: 10.1186/s10020-021-00299-y. Mol Med. 2021. PMID: 33858325 Free PMC article.
-
Role of pyroptosis in the pathogenesis of various neurological diseases.Brain Behav Immun. 2024 Mar;117:428-446. doi: 10.1016/j.bbi.2024.02.001. Epub 2024 Feb 7. Brain Behav Immun. 2024. PMID: 38336022 Review.
-
Pyroptosis: A Newly Discovered Therapeutic Target for Ischemia-Reperfusion Injury.Biomolecules. 2022 Nov 3;12(11):1625. doi: 10.3390/biom12111625. Biomolecules. 2022. PMID: 36358975 Free PMC article. Review.
-
Autophagy May Allow a Cell to Forbear Pyroptosis When Confronted With Cytosol-Invasive Bacteria.Front Immunol. 2022 Mar 29;13:871190. doi: 10.3389/fimmu.2022.871190. eCollection 2022. Front Immunol. 2022. PMID: 35422805 Free PMC article. Review.
References
-
- Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–454. - PubMed
-
- Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. - PubMed
-
- Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. - PubMed
-
- Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci. 2007;120:772–781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

