Photoactivated composite biomaterial for soft tissue restoration in rodents and in humans
- PMID: 21795587
- PMCID: PMC4652657
- DOI: 10.1126/scitranslmed.3002331
Photoactivated composite biomaterial for soft tissue restoration in rodents and in humans
Abstract
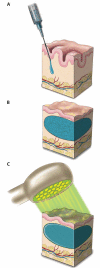
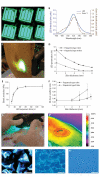
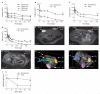
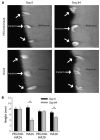
Soft tissue reconstruction often requires multiple surgical procedures that can result in scars and disfiguration. Facial soft tissue reconstruction represents a clinical challenge because even subtle deformities can severely affect an individual's social and psychological function. We therefore developed a biosynthetic soft tissue replacement composed of poly(ethylene glycol) (PEG) and hyaluronic acid (HA) that can be injected and photocrosslinked in situ with transdermal light exposure. Modulating the ratio of synthetic to biological polymer allowed us to tune implant elasticity and volume persistence. In a small-animal model, implanted photocrosslinked PEG-HA showed a dose-dependent relationship between increasing PEG concentration and enhanced implant volume persistence. In direct comparison with commercial HA injections, the PEG-HA implants maintained significantly greater average volumes and heights. Reversibility of the implant volume was achieved with hyaluronidase injection. Pilot clinical testing in human patients confirmed the feasibility of the transdermal photocrosslinking approach for implantation in abdomen soft tissue, although an inflammatory response was observed surrounding some of the materials.
Figures


Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Platelet-rich therapies for musculoskeletal soft tissue injuries.Cochrane Database Syst Rev. 2014 Apr 29;2014(4):CD010071. doi: 10.1002/14651858.CD010071.pub3. Cochrane Database Syst Rev. 2014. PMID: 24782334 Free PMC article. Review.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4. Cochrane Database Syst Rev. 2020. PMID: 32851663 Free PMC article.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Aug 27;8:CD007807. doi: 10.1002/14651858.CD007807.pub4. PMID: 28752910 Free PMC article. Updated. Review.
-
Behavioural interventions to treat anxiety in adults with autism and moderate to severe intellectual disabilities: the BEAMS-ID feasibility study.Health Technol Assess. 2024 Oct;28(72):1-147. doi: 10.3310/MWTQ5721. Health Technol Assess. 2024. PMID: 39487624 Free PMC article.
Cited by
-
Gellan gum microgel-reinforced cell-laden gelatin hydrogels.J Mater Chem B. 2014 May 7;2(17):2508-2516. doi: 10.1039/C3TB20984A. J Mater Chem B. 2014. PMID: 25309744 Free PMC article.
-
Dynamic and Cell-Infiltratable Hydrogels as Injectable Carrier of Therapeutic Cells and Drugs for Treating Challenging Bone Defects.ACS Cent Sci. 2019 Mar 27;5(3):440-450. doi: 10.1021/acscentsci.8b00764. Epub 2019 Feb 13. ACS Cent Sci. 2019. PMID: 30937371 Free PMC article.
-
Biodegradable and Non-Biodegradable Biomaterials and Their Effect on Cell Differentiation.Int J Mol Sci. 2022 Dec 19;23(24):16185. doi: 10.3390/ijms232416185. Int J Mol Sci. 2022. PMID: 36555829 Free PMC article. Review.
-
A perspective on the clinical translation of scaffolds for tissue engineering.Ann Biomed Eng. 2015 Mar;43(3):641-56. doi: 10.1007/s10439-014-1104-7. Epub 2014 Sep 9. Ann Biomed Eng. 2015. PMID: 25201605 Free PMC article.
-
ECM-incorporated hydrogels cross-linked via native chemical ligation to engineer stem cell microenvironments.Biomacromolecules. 2013 Sep 9;14(9):3102-11. doi: 10.1021/bm400728e. Epub 2013 Aug 20. Biomacromolecules. 2013. PMID: 23875943 Free PMC article.
References
-
- Robinson E, Rumsey N, Partridge J. An evaluation of the impact of social interaction skills training for facially disfigured people. Br. J. Plast. Surg. 1996;49:281–289. - PubMed
-
- Lee JC, St-Hilaire H, Christy MR, Wise MW, Rodriguez ED. Anterolateral thigh flap for trauma reconstruction. Ann. Plast. Surg. 2010;64:164–168. - PubMed
-
- Salles AG, Lotierzo PH, Gemperli R, Besteiro JM, Ishida LC, Gimenez RP, Menezes J, Ferreira MC. Complications after polymethylmethacrylate injections: Report of 32 cases. Plast. Reconstr. Surg. 2008;121:1811–1820. - PubMed
-
- Yoshimura K, Sato K, Aoi N, Kurita M, Inoue K, Suga H, Eto H, Kato H, Hirohi T, Harii K. Cell-assisted lipotransfer for facial lipoatrophy: Efficacy of clinical use of adipose-derived stem cells. Dermatol. Surg. 2008;34:1178–1185. - PubMed
-
- Seliktar D. Extracellular stimulation in tissue engineering. Ann. N. Y. Acad. Sci. 2005;1047:386–394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

