Vaccinia extracellular virions enter cells by macropinocytosis and acid-activated membrane rupture
- PMID: 21792173
- PMCID: PMC3181475
- DOI: 10.1038/emboj.2011.245
Vaccinia extracellular virions enter cells by macropinocytosis and acid-activated membrane rupture
Abstract
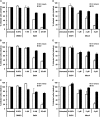
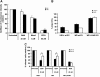
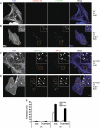
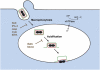
Vaccinia virus (VACV), the model poxvirus, produces two types of infectious particles: mature virions (MVs) and extracellular virions (EVs). EV particles possess two membranes and therefore require an unusual cellular entry mechanism. By a combination of fluorescence and electron microscopy as well as flow cytometry, we investigated the cellular processes that EVs required to infect HeLa cells. We found that EV particles were endocytosed, and that internalization and infection depended on actin rearrangements, activity of Na(+)/H(+) exchangers, and signalling events typical for the macropinocytic mechanism of endocytosis. To promote their internalization, EVs were capable of actively triggering macropinocytosis. EV infection also required vacuolar acidification, and acid exposure in endocytic vacuoles was needed to disrupt the outer EV membrane. Once exposed, the underlying MV-like particle presumably fused its single membrane with the limiting vacuolar membrane. Release of the viral core into the host cell cytosol allowed for productive infection.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Vaccinia virus strains use distinct forms of macropinocytosis for host-cell entry.Proc Natl Acad Sci U S A. 2010 May 18;107(20):9346-51. doi: 10.1073/pnas.1004618107. Epub 2010 May 3. Proc Natl Acad Sci U S A. 2010. PMID: 20439710 Free PMC article.
-
Resistance of a vaccinia virus A34R deletion mutant to spontaneous rupture of the outer membrane of progeny virions on the surface of infected cells.Virology. 2007 Sep 30;366(2):424-32. doi: 10.1016/j.virol.2007.05.015. Epub 2007 Jun 5. Virology. 2007. PMID: 17553539 Free PMC article.
-
Vaccinia virus A25 and A26 proteins are fusion suppressors for mature virions and determine strain-specific virus entry pathways into HeLa, CHO-K1, and L cells.J Virol. 2010 Sep;84(17):8422-32. doi: 10.1128/JVI.00599-10. Epub 2010 Jun 10. J Virol. 2010. PMID: 20538855 Free PMC article.
-
Poxvirus host cell entry.Curr Opin Virol. 2012 Feb;2(1):20-7. doi: 10.1016/j.coviro.2011.11.007. Epub 2011 Dec 27. Curr Opin Virol. 2012. PMID: 22440962 Review.
-
Virus entry by macropinocytosis.Nat Cell Biol. 2009 May;11(5):510-20. doi: 10.1038/ncb0509-510. Nat Cell Biol. 2009. PMID: 19404330 Review.
Cited by
-
Protein B5 is required on extracellular enveloped vaccinia virus for repulsion of superinfecting virions.J Gen Virol. 2012 Sep;93(Pt 9):1876-1886. doi: 10.1099/vir.0.043943-0. Epub 2012 May 23. J Gen Virol. 2012. PMID: 22622330 Free PMC article.
-
African swine fever virus-cell interactions: from virus entry to cell survival.Virus Res. 2013 Apr;173(1):42-57. doi: 10.1016/j.virusres.2012.12.006. Epub 2012 Dec 20. Virus Res. 2013. PMID: 23262167 Free PMC article. Review.
-
Vaccinia Virus Phospholipase Protein F13 Promotes Rapid Entry of Extracellular Virions into Cells.J Virol. 2018 May 14;92(11):e02154-17. doi: 10.1128/JVI.02154-17. Print 2018 Jun 1. J Virol. 2018. PMID: 29540596 Free PMC article.
-
ICAM-1 Binding Rhinoviruses Enter HeLa Cells via Multiple Pathways and Travel to Distinct Intracellular Compartments for Uncoating.Viruses. 2017 Apr 1;9(4):68. doi: 10.3390/v9040068. Viruses. 2017. PMID: 28368306 Free PMC article.
-
Mechanisms of Entry and Endosomal Pathway of African Swine Fever Virus.Vaccines (Basel). 2017 Nov 8;5(4):42. doi: 10.3390/vaccines5040042. Vaccines (Basel). 2017. PMID: 29117102 Free PMC article. Review.
References
-
- Chakrabarti S, Sisler JR, Moss B (1997) Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques 23: 1094–1097 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

