Insulin like growth factor-I: a critical mediator of the skeletal response to parathyroid hormone
- PMID: 21787292
- PMCID: PMC3690777
Insulin like growth factor-I: a critical mediator of the skeletal response to parathyroid hormone
Abstract
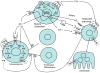
This review focuses on the mechanisms by which PTH stimulates both osteoblast and osteoclast function, emphasizing the critical role that IGF-I plays in these processes. After reviewing the current literature on the skeletal actions of PTH and the modulation of IGF action on bone by the different IGF-binding proteins, the review then examines studies from mouse models in which IGF-I or its receptor have been selectively deleted in different cells of the skeletal system, in particular osteoprogenitors, mature osteoblasts, and osteoclasts. Mice in which IGF-I production has been deleted from all cells are deficient in both bone formation and bone resorption with few osteoblasts or osteoclasts in bone in vivo, reduced osteoblast colony forming units, and an inability of either the osteoblasts or osteoclast precursors to support osteoclastogenesis in vitro. Mice in which the IGF-I receptor is specifically deleted in mature osteoblasts have a mineralization defect in vivo, and bone marrow stromal cells from these mice fail to mineralize in vitro. Mice in which the IGF-I receptor is deleted in osteoprogenitor cells have a marked reduction in osteoblast proliferation and differentiation leading to osteopenia. Finally mice lacking the IGF-I receptor in their osteoclasts have increased bone and decreased osteoclast formation. PTH fails to stimulate bone formation in the mice lacking IGF-I or its receptor in osteoblasts or enhance the signaling between osteoblasts and osteoclasts through RANKL/RANK and Ephrin B2/Eph B4, emphasizing the role IGF-I signaling plays in cell-communication per se and as stimulated by PTH.
Conflict of interest statement
None.
Figures
Similar articles
-
The Proteasome Inhibitor Carfilzomib Suppresses Parathyroid Hormone-induced Osteoclastogenesis through a RANKL-mediated Signaling Pathway.J Biol Chem. 2015 Jul 3;290(27):16918-28. doi: 10.1074/jbc.M115.663963. Epub 2015 May 15. J Biol Chem. 2015. PMID: 25979341 Free PMC article.
-
Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand.Bone. 1999 Nov;25(5):517-23. doi: 10.1016/s8756-3282(99)00210-0. Bone. 1999. PMID: 10574571
-
Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation.J Bone Miner Res. 2004 Nov;19(11):1873-81. doi: 10.1359/JBMR.040807. Epub 2004 Aug 16. J Bone Miner Res. 2004. PMID: 15476588
-
Osteoclast differentiation by RANKL and OPG signaling pathways.J Bone Miner Metab. 2021 Jan;39(1):19-26. doi: 10.1007/s00774-020-01162-6. Epub 2020 Oct 20. J Bone Miner Metab. 2021. PMID: 33079279 Review.
-
The insulin-like growth factor system and the coupling of formation to resorption.Bone. 1995 Aug;17(2 Suppl):93S-98S. doi: 10.1016/8756-3282(95)00186-h. Bone. 1995. PMID: 8579905 Review.
Cited by
-
Lactoferrin promote primary rat osteoblast proliferation and differentiation via up-regulation of insulin-like growth factor-1 expression.Mol Biol Rep. 2014 Aug;41(8):5019-30. doi: 10.1007/s11033-014-3368-2. Epub 2014 May 3. Mol Biol Rep. 2014. Retraction in: Mol Biol Rep. 2015 Oct;42(10):1467. doi: 10.1007/s11033-015-3897-3. PMID: 24792235 Retracted.
-
The calcium channel TRPV6 is a novel regulator of RANKL-induced osteoclastic differentiation and bone absorption activity through the IGF-PI3K-AKT pathway.Cell Prolif. 2021 Jan;54(1):e12955. doi: 10.1111/cpr.12955. Epub 2020 Nov 7. Cell Prolif. 2021. PMID: 33159483 Free PMC article.
-
Eph-Ephrin Signaling Mediates Cross-Talk Within the Bone Microenvironment.Front Cell Dev Biol. 2021 Feb 9;9:598612. doi: 10.3389/fcell.2021.598612. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33634116 Free PMC article. Review.
-
Sexual maturation of HIV-infected and uninfected male children in Abakaliki, South-East, Nigeria: a cross-sectional study.Pan Afr Med J. 2022 Jun 17;42:133. doi: 10.11604/pamj.2022.42.133.29305. eCollection 2022. Pan Afr Med J. 2022. PMID: 36060839 Free PMC article.
-
Genome-Wide Mapping and Interrogation of the Nmp4 Antianabolic Bone Axis.Mol Endocrinol. 2015 Sep;29(9):1269-85. doi: 10.1210/me.2014-1406. Epub 2015 Aug 5. Mol Endocrinol. 2015. PMID: 26244796 Free PMC article.
References
-
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. - PubMed
-
- Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. - PubMed
-
- Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349:1216–1226. - PubMed
-
- Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, Chandrasekhar S, Martin TJ, Onyia JE. Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142:4047–4054. - PubMed
-
- Frolik CA, Black EC, Cain RL, Satterwhite JH, Brown-Augsburger PL, Sato M, Hock JM. Anabolic and catabolic bone effects of human parathyroid hormone (1-34) are predicted by duration of hormone exposure. Bone. 2003;33:372–379. - PubMed

