Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-κB
- PMID: 21771249
- PMCID: PMC5028794
- DOI: 10.1111/j.1474-9726.2011.00734.x
Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-κB
Erratum in
-
Erratum.Aging Cell. 2016 Oct;15(5):981-2. doi: 10.1111/acel.12457. Aging Cell. 2016. PMID: 27599757 Free PMC article. No abstract available.
Abstract
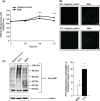
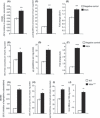
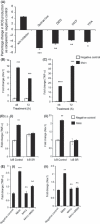
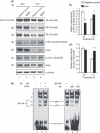
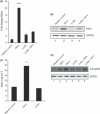
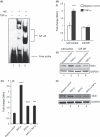
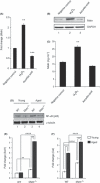
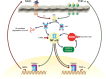
Abnormal levels of reactive oxygen species (ROS) and inflammatory cytokines have been observed in the skeletal muscle during muscle wasting including sarcopenia. However, the mechanisms that signal ROS production and prolonged maintenance of ROS levels during muscle wasting are not fully understood. Here, we show that myostatin (Mstn) is a pro-oxidant and signals the generation of ROS in muscle cells. Myostatin, a transforming growth factor-β (TGF-β) family member, has been shown to play an important role in skeletal muscle wasting by increasing protein degradation. Our results here show that Mstn induces oxidative stress by producing ROS in skeletal muscle cells through tumor necrosis factor-α (TNF-α) signaling via NF-κB and NADPH oxidase. Aged Mstn null (Mstn(-/-) ) muscles, which display reduced sarcopenia, also show an increased basal antioxidant enzyme (AOE) levels and lower NF-κB levels indicating efficient scavenging of excess ROS. Additionally, our results indicate that both TNF-α and hydrogen peroxide (H(2) O(2) ) are potent inducers of Mstn and require NF-κB signaling for Mstn induction. These results demonstrate that Mstn and TNF-α are components of a feed forward loop in which Mstn triggers the generation of second messenger ROS, mediated by TNF-α and NADPH oxidase, and the elevated TNF-α in turn stimulates Mstn expression. Higher levels of Mstn in turn induce muscle wasting by activating proteasomal-mediated catabolism of intracellular proteins. Thus, we propose that inhibition of ROS induced by Mstn could lead to reduced muscle wasting during sarcopenia.
© 2011 The Authors. Aging Cell © 2011 Blackwell Publishing Ltd/Anatomical Society of Great Britain and Ireland.
Figures

Similar articles
-
Exercise training decreases NADPH oxidase activity and restores skeletal muscle mass in heart failure rats.J Appl Physiol (1985). 2017 Apr 1;122(4):817-827. doi: 10.1152/japplphysiol.00182.2016. Epub 2017 Jan 19. J Appl Physiol (1985). 2017. PMID: 28104751
-
Interaction of Fibromodulin and Myostatin to Regulate Skeletal Muscle Aging: An Opposite Regulation in Muscle Aging, Diabetes, and Intracellular Lipid Accumulation.Cells. 2021 Aug 13;10(8):2083. doi: 10.3390/cells10082083. Cells. 2021. PMID: 34440852 Free PMC article.
-
Myostatin is a novel tumoral factor that induces cancer cachexia.Biochem J. 2012 Aug 15;446(1):23-36. doi: 10.1042/BJ20112024. Biochem J. 2012. Retraction in: Biochem J. 2016 Apr 15;473(8):1111. doi: 10.1042/BJ4731111. PMID: 22621320 Free PMC article. Retracted.
-
Sarcopenia in Chronic Kidney Disease: Factors, Mechanisms, and Therapeutic Interventions.Biol Pharm Bull. 2019;42(9):1437-1445. doi: 10.1248/bpb.b19-00513. Biol Pharm Bull. 2019. PMID: 31474705 Review.
-
The role of TGF-β signaling in muscle atrophy, sarcopenia and cancer cachexia.Gen Comp Endocrinol. 2024 Jul 1;353:114513. doi: 10.1016/j.ygcen.2024.114513. Epub 2024 Apr 10. Gen Comp Endocrinol. 2024. PMID: 38604437 Review.
Cited by
-
Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses.Clin Liver Dis. 2012 Feb;16(1):95-131. doi: 10.1016/j.cld.2011.12.009. Epub 2012 Jan 23. Clin Liver Dis. 2012. PMID: 22321468 Free PMC article. Review.
-
Elevated hydrogen peroxide and decreased catalase and glutathione peroxidase protection are associated with aging sarcopenia.BMC Geriatr. 2013 Oct 7;13:104. doi: 10.1186/1471-2318-13-104. BMC Geriatr. 2013. PMID: 24093947 Free PMC article.
-
Transcriptional activation of follistatin by Nrf2 protects pulmonary epithelial cells against silica nanoparticle-induced oxidative stress.Sci Rep. 2016 Feb 16;6:21133. doi: 10.1038/srep21133. Sci Rep. 2016. PMID: 26878911 Free PMC article.
-
Alleviating exercise-induced muscular stress using neat and processed bee pollen: oxidative markers, mitochondrial enzymes, and myostatin expression in rats.Integr Med Res. 2015 Sep;4(3):147-160. doi: 10.1016/j.imr.2015.02.003. Epub 2015 Mar 14. Integr Med Res. 2015. PMID: 28664121 Free PMC article.
-
Increased levels of interleukin-6 exacerbate the dystrophic phenotype in mdx mice.Hum Mol Genet. 2015 Nov 1;24(21):6041-53. doi: 10.1093/hmg/ddv323. Epub 2015 Aug 6. Hum Mol Genet. 2015. PMID: 26251044 Free PMC article.
References
-
- Aiken J, Bua E, Cao Z, Lopez M, Wanagat JON, McKenzie D, McKiernan S (2002) Mitochondrial DNA deletion mutations and sarcopenia. Ann. N Y Acad. Sci. 959, 412–423. - PubMed
-
- Baumann AP, Ibebunjo C, Grasser WA, Paralkar VM (2003) Myostatin expression in age and denervation‐induced skeletal muscle atrophy. J. Musculoskelet. Neuronal Interact. 3, 8–16. - PubMed
-
- Beers RF, Sizer IW (1952) A Spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195, 133–140. - PubMed
-
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal. Biochem. 72, 248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

