Membrane shaping by the Bin/amphiphysin/Rvs (BAR) domain protein superfamily
- PMID: 21769645
- PMCID: PMC11114942
- DOI: 10.1007/s00018-011-0768-5
Membrane shaping by the Bin/amphiphysin/Rvs (BAR) domain protein superfamily
Abstract
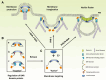
BAR domain superfamily proteins have emerged as central regulators of dynamic membrane remodeling, thereby playing important roles in a wide variety of cellular processes, such as organelle biogenesis, cell division, cell migration, secretion, and endocytosis. Here, we review the mechanistic and structural basis for the membrane curvature-sensing and deforming properties of BAR domain superfamily proteins. Moreover, we summarize the present state of knowledge with respect to their regulation by autoinhibitory mechanisms or posttranslational modifications, and their interactions with other proteins, in particular with GTPases, and with membrane lipids. We postulate that BAR superfamily proteins act as membrane-deforming scaffolds that spatiotemporally orchestrate membrane remodeling.
Figures

Similar articles
-
F-BAR family proteins, emerging regulators for cell membrane dynamic changes-from structure to human diseases.J Hematol Oncol. 2015 May 9;8:47. doi: 10.1186/s13045-015-0144-2. J Hematol Oncol. 2015. PMID: 25956236 Free PMC article. Review.
-
Membrane re-modelling by BAR domain superfamily proteins via molecular and non-molecular factors.Biochem Soc Trans. 2018 Apr 17;46(2):379-389. doi: 10.1042/BST20170322. Epub 2018 Mar 14. Biochem Soc Trans. 2018. PMID: 29540508 Review.
-
Roles of amphipathic helices and the bin/amphiphysin/rvs (BAR) domain of endophilin in membrane curvature generation.J Biol Chem. 2010 Jun 25;285(26):20164-70. doi: 10.1074/jbc.M110.127811. Epub 2010 Apr 23. J Biol Chem. 2010. PMID: 20418375 Free PMC article.
-
BAR domain scaffolds in dynamin-mediated membrane fission.Cell. 2014 Feb 27;156(5):882-92. doi: 10.1016/j.cell.2014.02.017. Cell. 2014. PMID: 24581490 Review.
-
Structural characteristics of BAR domain superfamily to sculpt the membrane.Semin Cell Dev Biol. 2010 Jun;21(4):391-8. doi: 10.1016/j.semcdb.2010.01.010. Epub 2010 Jan 18. Semin Cell Dev Biol. 2010. PMID: 20083215 Review.
Cited by
-
Analysis of diffusion in curved surfaces and its application to tubular membranes.Mol Biol Cell. 2016 Dec 1;27(24):3937-3946. doi: 10.1091/mbc.E16-06-0445. Epub 2016 Oct 12. Mol Biol Cell. 2016. PMID: 27733625 Free PMC article.
-
Membrane curvature at a glance.J Cell Sci. 2015 Mar 15;128(6):1065-70. doi: 10.1242/jcs.114454. J Cell Sci. 2015. PMID: 25774051 Free PMC article. Review.
-
Emerging methodologies to investigate lipid-protein interactions.Integr Biol (Camb). 2012 Mar;4(3):247-58. doi: 10.1039/c2ib00143h. Epub 2012 Feb 10. Integr Biol (Camb). 2012. PMID: 22327461 Free PMC article. Review.
-
Cellular and molecular interactions of phosphoinositides and peripheral proteins.Chem Phys Lipids. 2014 Sep;182:3-18. doi: 10.1016/j.chemphyslip.2014.02.002. Epub 2014 Feb 17. Chem Phys Lipids. 2014. PMID: 24556335 Free PMC article. Review.
-
Clathrin-adaptor ratio and membrane tension regulate the flat-to-curved transition of the clathrin coat during endocytosis.Nat Commun. 2018 Mar 16;9(1):1109. doi: 10.1038/s41467-018-03533-0. Nat Commun. 2018. PMID: 29549258 Free PMC article.
References
-
- McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. - PubMed
-
- Lim KB, Bu W, Goh WI, Koh E, Ong SH, Pawson T, Sudhaharan T, Ahmed S. The Cdc42 effector IRSp53 generates filopodia by coupling membrane protrusion with actin dynamics. J Biol Chem. 2008;283:20454–20472. - PubMed
-
- Krugmann S, Jordens I, Gevaert K, Driessens M, Vandekerckhove J, Hall A. Cdc42 induces filopodia by promoting the formation of an IRSp53:Mena complex. Curr Biol. 2001;11:1645–1655. - PubMed
-
- Kamioka Y, Fukuhara S, Sawa H, Nagashima K, Masuda M, Matsuda M, Mochizuki N. A novel dynamin-associating molecule, formin-binding protein 17, induces tubular membrane invaginations and participates in endocytosis. J Biol Chem. 2004;279:40091–40099. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

