The kinase IKKα inhibits activation of the transcription factor NF-κB by phosphorylating the regulatory molecule TAX1BP1
- PMID: 21765415
- PMCID: PMC3205447
- DOI: 10.1038/ni.2066
The kinase IKKα inhibits activation of the transcription factor NF-κB by phosphorylating the regulatory molecule TAX1BP1
Abstract
In response to stimulation with proinflammatory cytokines, the deubiquitinase A20 inducibly interacts with the regulatory molecules TAX1BP1, Itch and RNF11 to form the A20 ubiquitin-editing complex. However, the molecular signal that coordinates the assembly of this complex has remained elusive. Here we demonstrate that TAX1BP1 was inducibly phosphorylated on Ser593 and Ser624 in response to proinflammatory stimuli. The kinase IKKα, but not IKKβ, was required for phosphorylation of TAX1BP1 and directly phosphorylated TAX1BP1 in response to stimulation with tumor necrosis factor (TNF) or interleukin 1 (IL-1). TAX1BP1 phosphorylation was pivotal for cytokine-dependent interactions among TAX1BP1, A20, Itch and RNF11 and downregulation of signaling by the transcription factor NF-κB. IKKα therefore serves a key role in the negative feedback of NF-κB canonical signaling by orchestrating assembly of the A20 ubiquitin-editing complex to limit inflammatory gene activation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
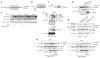






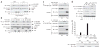
Comment in
-
IKKα takes control of canonical NF-κB activation.Nat Immunol. 2011 Aug 18;12(9):815-6. doi: 10.1038/ni.2082. Nat Immunol. 2011. PMID: 21852778 No abstract available.
Similar articles
-
Regulation of NF-κB signaling by the A20 deubiquitinase.Cell Mol Immunol. 2012 Mar;9(2):123-30. doi: 10.1038/cmi.2011.59. Epub 2012 Feb 20. Cell Mol Immunol. 2012. PMID: 22343828 Free PMC article. Review.
-
IKKα takes control of canonical NF-κB activation.Nat Immunol. 2011 Aug 18;12(9):815-6. doi: 10.1038/ni.2082. Nat Immunol. 2011. PMID: 21852778 No abstract available.
-
Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes.Science. 2010 Feb 26;327(5969):1135-9. doi: 10.1126/science.1182364. Science. 2010. PMID: 20185725 Free PMC article.
-
The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-kappaB signalling.EMBO J. 2009 Mar 4;28(5):513-22. doi: 10.1038/emboj.2008.285. Epub 2009 Jan 8. EMBO J. 2009. PMID: 19131965 Free PMC article.
-
A20-mediated negative regulation of canonical NF-κB signaling pathway.Immunol Res. 2013 Dec;57(1-3):166-71. doi: 10.1007/s12026-013-8463-2. Immunol Res. 2013. PMID: 24242761 Review.
Cited by
-
Regulation of B cell differentiation by the ubiquitin-binding protein TAX1BP1.Sci Rep. 2016 Aug 12;6:31266. doi: 10.1038/srep31266. Sci Rep. 2016. PMID: 27515252 Free PMC article.
-
Regulation of NF-κB signaling by the A20 deubiquitinase.Cell Mol Immunol. 2012 Mar;9(2):123-30. doi: 10.1038/cmi.2011.59. Epub 2012 Feb 20. Cell Mol Immunol. 2012. PMID: 22343828 Free PMC article. Review.
-
The long non-coding RNA SNHG1 attenuates chondrocyte apoptosis and inflammation via the miR-195/IKK-α axis.Cell Tissue Bank. 2023 Mar;24(1):167-180. doi: 10.1007/s10561-022-10019-3. Epub 2022 Jul 7. Cell Tissue Bank. 2023. PMID: 35796880
-
ABIN1 protein cooperates with TAX1BP1 and A20 proteins to inhibit antiviral signaling.J Biol Chem. 2011 Oct 21;286(42):36592-602. doi: 10.1074/jbc.M111.283762. Epub 2011 Sep 1. J Biol Chem. 2011. PMID: 21885437 Free PMC article.
-
Bone marrow-specific knock-in of a non-activatable Ikkα kinase mutant influences haematopoiesis but not atherosclerosis in Apoe-deficient mice.PLoS One. 2014 Feb 3;9(2):e87452. doi: 10.1371/journal.pone.0087452. eCollection 2014. PLoS One. 2014. PMID: 24498325 Free PMC article.
References
-
- Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. - PubMed
-
- Ghosh S, Hayden MS. New regulators of NF-κB in inflammation. Nat Rev Immunol. 2008;8:837–848. - PubMed
-
- Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. - PubMed
-
- Yamaoka S, et al. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell. 1998;93:1231–1240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

