MARCH ubiquitin ligases alter the itinerary of clathrin-independent cargo from recycling to degradation
- PMID: 21757542
- PMCID: PMC3164467
- DOI: 10.1091/mbc.E10-11-0874
MARCH ubiquitin ligases alter the itinerary of clathrin-independent cargo from recycling to degradation
Abstract
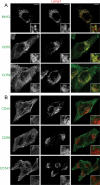
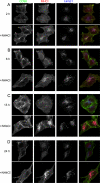
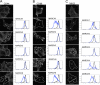
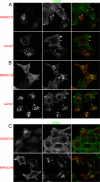
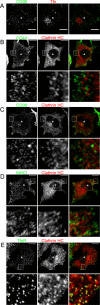
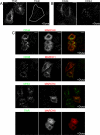
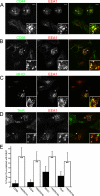
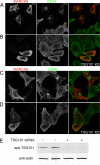
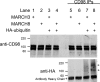
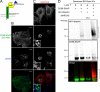
Following endocytosis, internalized plasma membrane proteins can be recycled back to the cell surface or trafficked to late endosomes/lysosomes for degradation. Here we report on the trafficking of multiple proteins that enter cells by clathrin-independent endocytosis (CIE) and determine that a set of proteins (CD44, CD98, and CD147) found primarily in recycling tubules largely failed to reach late endosomes in HeLa cells, whereas other CIE cargo proteins, including major histocompatibility complex class I protein (MHCI), trafficked to both early endosome antigen 1 (EEA1) and late endosomal compartments in addition to recycling tubules. Expression of the membrane-associated RING-CH 8 (MARCH8) E3 ubiquitin ligase completely shifted the trafficking of CD44 and CD98 proteins away from recycling tubules to EEA1 compartments and late endosomes, resulting in reduced surface levels. Cargo affected by MARCH expression, including CD44, CD98, and MHCI, still entered cells by CIE, suggesting that the routing of ubiquitinated cargo occurs after endocytosis. MARCH8 expression led to direct ubiquitination of CD98 and routing of CD98 to late endosomes/lysosomes.
Figures
Similar articles
-
Hook1, microtubules, and Rab22: mediators of selective sorting of clathrin-independent endocytic cargo proteins on endosomes.Bioarchitecture. 2013 Sep-Dec;3(5):141-6. doi: 10.4161/bioa.26638. Epub 2013 Sep 1. Bioarchitecture. 2013. PMID: 24284901 Free PMC article.
-
Microtubule-dependent endosomal sorting of clathrin-independent cargo by Hook1.J Cell Biol. 2013 Apr 15;201(2):233-47. doi: 10.1083/jcb.201208172. J Cell Biol. 2013. PMID: 23589492 Free PMC article.
-
Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis.Traffic. 2009 May;10(5):590-9. doi: 10.1111/j.1600-0854.2009.00894.x. Traffic. 2009. PMID: 19302270 Free PMC article.
-
Ubiquitin and endocytic protein sorting.Essays Biochem. 2005;41:81-98. doi: 10.1042/EB0410081. Essays Biochem. 2005. PMID: 16250899 Review.
-
Endosomal transport via ubiquitination.Trends Cell Biol. 2011 Nov;21(11):647-55. doi: 10.1016/j.tcb.2011.08.007. Epub 2011 Sep 28. Trends Cell Biol. 2011. PMID: 21955996 Free PMC article. Review.
Cited by
-
Advances in HIV-1 Assembly.Viruses. 2022 Feb 26;14(3):478. doi: 10.3390/v14030478. Viruses. 2022. PMID: 35336885 Free PMC article. Review.
-
CD44 Intracellular Domain: A Long Tale of a Short Tail.Cancers (Basel). 2023 Oct 18;15(20):5041. doi: 10.3390/cancers15205041. Cancers (Basel). 2023. PMID: 37894408 Free PMC article. Review.
-
Hook1, microtubules, and Rab22: mediators of selective sorting of clathrin-independent endocytic cargo proteins on endosomes.Bioarchitecture. 2013 Sep-Dec;3(5):141-6. doi: 10.4161/bioa.26638. Epub 2013 Sep 1. Bioarchitecture. 2013. PMID: 24284901 Free PMC article.
-
Ubiquitin-dependent sorting in endocytosis.Cold Spring Harb Perspect Biol. 2014 Jan 1;6(1):a016808. doi: 10.1101/cshperspect.a016808. Cold Spring Harb Perspect Biol. 2014. PMID: 24384571 Free PMC article. Review.
-
MARCH family E3 ubiquitin ligases selectively target and degrade cadherin family proteins.PLoS One. 2024 May 9;19(5):e0290485. doi: 10.1371/journal.pone.0290485. eCollection 2024. PLoS One. 2024. PMID: 38722959 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

