Sucrose counteracts the anti-inflammatory effect of fish oil in adipose tissue and increases obesity development in mice
- PMID: 21738749
- PMCID: PMC3125273
- DOI: 10.1371/journal.pone.0021647
Sucrose counteracts the anti-inflammatory effect of fish oil in adipose tissue and increases obesity development in mice
Abstract
Background: Polyunsaturated n-3 fatty acids (n-3 PUFAs) are reported to protect against high fat diet-induced obesity and inflammation in adipose tissue. Here we aimed to investigate if the amount of sucrose in the background diet influences the ability of n-3 PUFAs to protect against diet-induced obesity, adipose tissue inflammation and glucose intolerance.
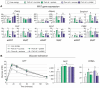
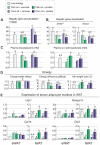
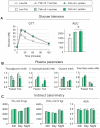
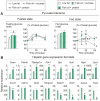
Methodology/principal findings: We fed C57BL/6J mice a protein- (casein) or sucrose-based high fat diet supplemented with fish oil or corn oil for 9 weeks. Irrespective of the fatty acid source, mice fed diets rich in sucrose became obese whereas mice fed high protein diets remained lean. Inclusion of sucrose in the diet also counteracted the well-known anti-inflammatory effect of fish oil in adipose tissue, but did not impair the ability of fish oil to prevent accumulation of fat in the liver. Calculation of HOMA-IR indicated that mice fed high levels of proteins remained insulin sensitive, whereas insulin sensitivity was reduced in the obese mice fed sucrose irrespectively of the fat source. We show that a high fat diet decreased glucose tolerance in the mice independently of both obesity and dietary levels of n-3 PUFAs and sucrose. Of note, increasing the protein∶sucrose ratio in high fat diets decreased energy efficiency irrespective of fat source. This was accompanied by increased expression of Ppargc1a (peroxisome proliferator-activated receptor, gamma, coactivator 1 alpha) and increased gluconeogenesis in the fed state.
Conclusions/significance: The background diet influence the ability of n-3 PUFAs to protect against development of obesity, glucose intolerance and adipose tissue inflammation. High levels of dietary sucrose counteract the anti-inflammatory effect of fish oil in adipose tissue and increases obesity development in mice.
Conflict of interest statement
Figures


Similar articles
-
High-glycemic index carbohydrates abrogate the antiobesity effect of fish oil in mice.Am J Physiol Endocrinol Metab. 2012 May 15;302(9):E1097-112. doi: 10.1152/ajpendo.00524.2011. Epub 2012 Feb 14. Am J Physiol Endocrinol Metab. 2012. PMID: 22338077
-
Low-Molecular-Weight Peptides from Salmon Protein Prevent Obesity-Linked Glucose Intolerance, Inflammation, and Dyslipidemia in LDLR-/-/ApoB100/100 Mice.J Nutr. 2015 Jul;145(7):1415-22. doi: 10.3945/jn.114.208215. Epub 2015 May 20. J Nutr. 2015. PMID: 25995281
-
Fish oil supplementation inhibits endoplasmic reticulum stress and improves insulin resistance: involvement of AMP-activated protein kinase.Food Funct. 2017 Apr 19;8(4):1481-1493. doi: 10.1039/c6fo01841f. Food Funct. 2017. PMID: 28327709
-
Interplay between fish oil, obesity and cardiometabolic diabetes.J Formos Med Assoc. 2023 Jul;122(7):528-539. doi: 10.1016/j.jfma.2023.03.013. Epub 2023 Mar 29. J Formos Med Assoc. 2023. PMID: 37002172 Review.
-
High-Fat Diet and Female Fertility.Endocrinology. 2017 Aug 1;158(8):2407-2419. doi: 10.1210/en.2017-00371. Endocrinology. 2017. PMID: 28586412 Free PMC article. Review.
Cited by
-
The Impact of Different Animal-Derived Protein Sources on Adiposity and Glucose Homeostasis during Ad Libitum Feeding and Energy Restriction in Already Obese Mice.Nutrients. 2019 May 23;11(5):1153. doi: 10.3390/nu11051153. Nutrients. 2019. PMID: 31126082 Free PMC article.
-
Of mice and men: Factors abrogating the antiobesity effect of omega-3 fatty acids.Adipocyte. 2012 Jul 1;1(3):173-176. doi: 10.4161/adip.20689. Adipocyte. 2012. PMID: 23700529 Free PMC article.
-
The Relationship between the Source of Dietary Animal Fats and Proteins and the Gut Microbiota Condition and Obesity in Humans.Nutrients. 2023 Jul 9;15(14):3082. doi: 10.3390/nu15143082. Nutrients. 2023. PMID: 37513500 Free PMC article. Review.
-
Dietary linoleic acid elevates the endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet.Lipids. 2014 Jan;49(1):59-69. doi: 10.1007/s11745-013-3842-y. Epub 2013 Oct 1. Lipids. 2014. PMID: 24081493 Free PMC article.
-
Scallop protein with endogenous high taurine and glycine content prevents high-fat, high-sucrose-induced obesity and improves plasma lipid profile in male C57BL/6J mice.Amino Acids. 2014 Jul;46(7):1659-71. doi: 10.1007/s00726-014-1715-1. Amino Acids. 2014. PMID: 24658997 Free PMC article.
References
-
- Erkkilä A, de Mello VDF, Risérus U, Laaksonen DE. Dietary fatty acids and cardiovascular disease: An epidemiological approach. Progress in Lipid Research. 2008;47:172–187. - PubMed
-
- Harris W. The Omega-3 Index: Clinical Utility for Therapeutic Intervention. Current Cardiology Reports. 2010;12:503–508. - PubMed
-
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedecine & Pharmacotherapy. 2002;56:365–379. - PubMed
-
- Ailhaud G, Massiera F, Weill P, Legrand P, Alessandri JM, et al. Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog Lipid Res. 2006;45:203–236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

