Proteomic analysis of short- and intermediate-term memory in Hermissenda
- PMID: 21736919
- PMCID: PMC3166390
- DOI: 10.1016/j.neuroscience.2011.06.063
Proteomic analysis of short- and intermediate-term memory in Hermissenda
Abstract
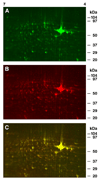
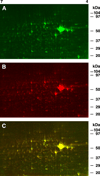
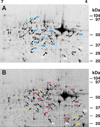
Changes in cellular and synaptic plasticity related to learning and memory are accompanied by both upregulation and downregulation of the expression levels of proteins. Both de novo protein synthesis and post-translational modification of existing proteins have been proposed to support the induction and maintenance of memory underlying learning. However, little is known regarding the identity of proteins regulated by learning that are associated with the early stages supporting the formation of memory over time. In this study we have examined changes in protein abundance at two different times following one-trial in vitro conditioning of Hermissenda using two-dimensional difference gel electrophoresis (2D-DIGE), quantification of differences in protein abundance between conditioned and unpaired controls, and protein identification with tandem mass spectrometry. Significant regulation of protein abundance following one-trial in vitro conditioning was detected 30 min and 3 h post-conditioning. Proteins were identified that exhibited statistically significant increased or decreased abundance at both 30 min and 3 h post-conditioning. Proteins were also identified that exhibited a significant increase in abundance only at 30 min, or only at 3 h post-conditioning. A few proteins were identified that expressed a significant decrease in abundance detected at both 30 min and 3 h post-conditioning, or a significant decrease in abundance only at 3 h post-conditioning. The proteomic analysis indicates that proteins involved in diverse cellular functions such as translational regulation, cell signaling, cytoskeletal regulation, metabolic activity, and protein degradation contribute to the formation of memory produced by one-trial in vitro conditioning. These findings support the view that changes in protein abundance over time following one-trial in vitro conditioning involve dynamic and complex interactions of the proteome.
Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Proteomic analysis of post-translational modifications in conditioned Hermissenda.Neuroscience. 2010 Feb 17;165(4):1182-90. doi: 10.1016/j.neuroscience.2009.11.066. Epub 2009 Dec 1. Neuroscience. 2010. PMID: 19961907 Free PMC article.
-
Identification of a 24 kDa phosphoprotein associated with an intermediate stage of memory in Hermissenda.J Neurosci. 2000 May 15;20(10):RC74. doi: 10.1523/JNEUROSCI.20-10-j0002.2000. J Neurosci. 2000. PMID: 10783398 Free PMC article.
-
Subcellular, cellular, and circuit mechanisms underlying classical conditioning in Hermissenda crassicornis.Anat Rec B New Anat. 2006 Jan;289(1):25-37. doi: 10.1002/ar.b.20090. Anat Rec B New Anat. 2006. PMID: 16437555 Free PMC article. Review.
-
Time-dependent increase in protein phosphorylation following one-trial enhancement in Hermissenda.J Neurochem. 1996 Apr;66(4):1736-41. doi: 10.1046/j.1471-4159.1996.66041736.x. J Neurochem. 1996. PMID: 8627332
-
Comparative study of visuo-vestibular conditioning in Lymnaea stagnalis.Biol Bull. 2006 Jun;210(3):298-307. doi: 10.2307/4134566. Biol Bull. 2006. PMID: 16801503 Review.
Cited by
-
Susceptibility of memory consolidation during lapses in recall.Nat Commun. 2013;4:1578. doi: 10.1038/ncomms2591. Nat Commun. 2013. PMID: 23481386 Free PMC article.
-
Critical period of memory enhancement during taste avoidance conditioning in Lymnaea stagnalis.PLoS One. 2013 Oct 3;8(10):e75276. doi: 10.1371/journal.pone.0075276. eCollection 2013. PLoS One. 2013. PMID: 24098373 Free PMC article.
References
-
- Alban A, David S, Bjorkesten L, Andersson C, Sloge E, Lewis S, Currie I. A novel experimental design for comparative two-dimensional gel analysis: Two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics. 2003;3:36–44. - PubMed
-
- Ascoli G, Liu KX, Olds JL, Nelson TJ, Gusov PA, Bertucci C, Bramanti E, Raffaelli A, Salvador P, Alkon DL. Secondary structure of Ca2+-induced conformational change of calexcitin, a learning associated protein. J Biol Chem. 1997;272:29771–29779. - PubMed
-
- Autieri MV, Kelemen SE, Wendt KW. AIF-1 is an actin-polymerizing and rac1-activating protein that promotes vascular smooth muscle cell migration. Cir Res. 2003;92:1107–1114. - PubMed
-
- Bailey DJ, Kim JJ, Sun W, Thompson RF, Helmstetter FJ. Acquisition of fear conditioning in rats requires the synthesis of mRNA in the amygdala. Behav Neurosci. 1999;113:276–282. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

