Interaction and interdependent packaging of tegument protein UL11 and glycoprotein e of herpes simplex virus
- PMID: 21734040
- PMCID: PMC3165753
- DOI: 10.1128/JVI.05207-11
Interaction and interdependent packaging of tegument protein UL11 and glycoprotein e of herpes simplex virus
Abstract
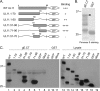
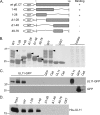
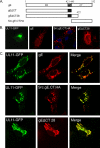
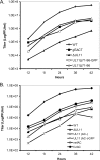
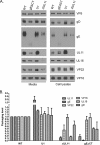
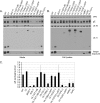
The UL11 tegument protein of herpes simplex virus plays a critical role in the secondary envelopment; however, the mechanistic details remain elusive. Here, we report a new function of UL11 in the budding process in which it directs efficient acquisition of glycoprotein E (gE) via a direct interaction. In vitro binding assays showed that the interaction required only the first 28, membrane-proximal residues of the cytoplasmic tail of gE, and the C-terminal 26 residues of UL11. A second, weaker binding site was also found in the N-terminal half of UL11. The significance of the gE-UL11 interaction was subsequently investigated with viral deletion mutants. In the absence of the gE tail, virion packaging of UL11, but not other tegument proteins such as VP22 and VP16, was reduced by at least 80%. Reciprocally, wild-type gE packaging was also drastically reduced by about 87% in the absence of UL11, and this defect could be rescued in trans by expressing U(L)11 at the U(L)35 locus. Surprisingly, a mutant that lacks the C-terminal gE-binding site of UL11 packaged nearly normal amounts of gE despite its strong interaction with the gE tail in vitro, indicating that the interaction with the UL11 N terminus may be important. Mutagenesis studies of the UL11 N terminus revealed that the association of UL11 with membrane was not required for this function. In contrast, the UL11 acidic cluster motif was found to be critical for gE packaging and was not replaceable with foreign acidic clusters. Together, these results highlight an important role of UL11 in the acquisition of glycoprotein-enriched lipid bilayers, and the findings may also have important implications for the role of UL11 in gE-mediated cell-to-cell spread.
Figures


Similar articles
-
Cytoplasmic residues of herpes simplex virus glycoprotein gE required for secondary envelopment and binding of tegument proteins VP22 and UL11 to gE and gD.J Virol. 2007 Jan;81(1):319-31. doi: 10.1128/JVI.01842-06. Epub 2006 Oct 11. J Virol. 2007. PMID: 17035313 Free PMC article.
-
Virion incorporation of the herpes simplex virus type 1 tegument protein VP22 is facilitated by trans-Golgi network localization and is independent of interaction with glycoprotein E.Virology. 2010 Sep 15;405(1):176-92. doi: 10.1016/j.virol.2010.06.007. Epub 2010 Jun 26. Virology. 2010. PMID: 20580397 Free PMC article.
-
Virion incorporation of the herpes simplex virus type 1 tegument protein VP22 occurs via glycoprotein E-specific recruitment to the late secretory pathway.J Virol. 2009 May;83(10):5204-18. doi: 10.1128/JVI.00069-09. Epub 2009 Mar 11. J Virol. 2009. PMID: 19279114 Free PMC article.
-
Features and Functions of the Conserved Herpesvirus Tegument Protein UL11 and Its Binding Partners.Front Microbiol. 2022 Jun 3;13:829754. doi: 10.3389/fmicb.2022.829754. eCollection 2022. Front Microbiol. 2022. PMID: 35722336 Free PMC article. Review.
-
Alphaherpesvirus glycoprotein E: A review of its interactions with other proteins of the virus and its application in vaccinology.Front Microbiol. 2022 Aug 4;13:970545. doi: 10.3389/fmicb.2022.970545. eCollection 2022. Front Microbiol. 2022. PMID: 35992696 Free PMC article. Review.
Cited by
-
The herpes simplex virus 1 UL51 protein interacts with the UL7 protein and plays a role in its recruitment into the virion.J Virol. 2015 Mar;89(6):3112-22. doi: 10.1128/JVI.02799-14. Epub 2014 Dec 31. J Virol. 2015. PMID: 25552711 Free PMC article.
-
Evaluation of safety and immunogenicity of duck-plague virus gC/gE double gene deletion.Front Immunol. 2022 Aug 18;13:963009. doi: 10.3389/fimmu.2022.963009. eCollection 2022. Front Immunol. 2022. PMID: 36059553 Free PMC article.
-
UL11 Protein Is a Key Participant of the Duck Plague Virus in Its Life Cycle.Front Microbiol. 2022 Jan 4;12:792361. doi: 10.3389/fmicb.2021.792361. eCollection 2021. Front Microbiol. 2022. PMID: 35058907 Free PMC article.
-
Proteomic characterization of murid herpesvirus 4 extracellular virions.PLoS One. 2013 Dec 30;8(12):e83842. doi: 10.1371/journal.pone.0083842. eCollection 2013. PLoS One. 2013. PMID: 24386290 Free PMC article.
-
Evolution and diversity in human herpes simplex virus genomes.J Virol. 2014 Jan;88(2):1209-27. doi: 10.1128/JVI.01987-13. Epub 2013 Nov 13. J Virol. 2014. PMID: 24227835 Free PMC article.
References
-
- Basu S., Dubin G., Basu M., Nguyen V., Friedman H. M. 1995. Characterization of regions of herpes simplex virus type 1 glycoprotein E involved in binding the Fc domain of monomeric IgG and in forming a complex with glycoprotein I. J. Immunol. 154:260–267 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

