Afadin controls p120-catenin-ZO-1 interactions leading to endothelial barrier enhancement by oxidized phospholipids
- PMID: 21732359
- PMCID: PMC3659796
- DOI: 10.1002/jcp.22916
Afadin controls p120-catenin-ZO-1 interactions leading to endothelial barrier enhancement by oxidized phospholipids
Abstract
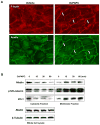
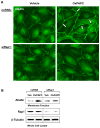
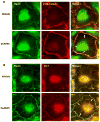
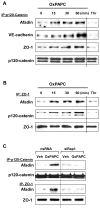
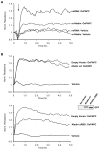
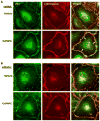
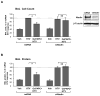
Afadin is a novel regulator of epithelial cell junctions assembly. However, its role in the formation of endothelial cell junctions and the regulation of vascular permeability remains obscure. We previously described protective effects of oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC) in the in vitro and in vivo models of lung endothelial barrier dysfunction and acute lung injury, which were mediated by Rac GTPase. This study examined a role of afadin in the OxPAPC-induced enhancement of interactions between adherens junctions and tight junctions as a novel mechanism of endothelial cell (EC) barrier preservation. OxPAPC induced Rap1-dependent afadin accumulation at the cell periphery and Rap1-dependent afadin interaction with adherens junction and tight junction proteins p120-catenin and ZO-1, respectively. Afadin knockdown using siRNA or ectopic expression of afadin mutant lacking Rap1 GTPase binding domain suppressed OxPAPC-induced EC barrier enhancement and abolished barrier protective effects of OxPAPC against thrombin-induced EC permeability. Afadin knockdown also abolished protective effects of OxPAPC against ventilator-induced lung injury in vivo. These results demonstrate for the first time a critical role of afadin in the regulation of vascular barrier function in vitro and in vivo via coordination of adherens junction-tight junction interactions.
Copyright © 2011 Wiley Periodicals, Inc.
Figures


Similar articles
-
Association between adherens junctions and tight junctions via Rap1 promotes barrier protective effects of oxidized phospholipids.J Cell Physiol. 2011 Aug;226(8):2052-62. doi: 10.1002/jcp.22543. J Cell Physiol. 2011. PMID: 21520057 Free PMC article.
-
p190RhoGAP mediates protective effects of oxidized phospholipids in the models of ventilator-induced lung injury.Exp Cell Res. 2011 Apr 1;317(6):859-72. doi: 10.1016/j.yexcr.2010.11.011. Epub 2010 Nov 25. Exp Cell Res. 2011. PMID: 21111731 Free PMC article.
-
Paxillin-beta-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids.Am J Physiol Lung Cell Mol Physiol. 2007 Jul;293(1):L199-211. doi: 10.1152/ajplung.00020.2007. Epub 2007 May 18. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17513457
-
Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions.Ann N Y Acad Sci. 2009 May;1165:113-20. doi: 10.1111/j.1749-6632.2009.04440.x. Ann N Y Acad Sci. 2009. PMID: 19538295 Free PMC article. Review.
-
Rap1 in endothelial biology.Curr Opin Hematol. 2017 May;24(3):248-255. doi: 10.1097/MOH.0000000000000332. Curr Opin Hematol. 2017. PMID: 28178039 Free PMC article. Review.
Cited by
-
Prostacyclin post-treatment improves LPS-induced acute lung injury and endothelial barrier recovery via Rap1.Biochim Biophys Acta. 2015 May;1852(5):778-91. doi: 10.1016/j.bbadis.2014.12.016. Epub 2014 Dec 26. Biochim Biophys Acta. 2015. PMID: 25545047 Free PMC article.
-
Oxidized Phospholipids in Control of Endothelial Barrier Function: Mechanisms and Implication in Lung Injury.Front Endocrinol (Lausanne). 2021 Nov 23;12:794437. doi: 10.3389/fendo.2021.794437. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34887839 Free PMC article. Review.
-
Tight Junctions, the Epithelial Barrier, and Toll-like Receptor-4 During Lung Injury.Inflammation. 2022 Dec;45(6):2142-2162. doi: 10.1007/s10753-022-01708-y. Epub 2022 Jul 2. Inflammation. 2022. PMID: 35779195 Free PMC article. Review.
-
Anti-Inflammatory Effects of OxPAPC Involve Endothelial Cell-Mediated Generation of LXA4.Circ Res. 2017 Jul 21;121(3):244-257. doi: 10.1161/CIRCRESAHA.116.310308. Epub 2017 May 18. Circ Res. 2017. PMID: 28522438 Free PMC article.
-
Suppression of SIPA-1 expression may reduce bladder cancer invasion and metastasis via the downregulation of E-cadherin and ZO-1.Exp Ther Med. 2016 Jan;11(1):213-217. doi: 10.3892/etm.2015.2891. Epub 2015 Nov 24. Exp Ther Med. 2016. PMID: 26889242 Free PMC article.
References
-
- Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95(9):892–901. - PubMed
-
- Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, Birukov KG. Polar head groups are important for barrier protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2007b;292(4):L924–935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

