Silymarin protects epidermal keratinocytes from ultraviolet radiation-induced apoptosis and DNA damage by nucleotide excision repair mechanism
- PMID: 21731736
- PMCID: PMC3120878
- DOI: 10.1371/journal.pone.0021410
Silymarin protects epidermal keratinocytes from ultraviolet radiation-induced apoptosis and DNA damage by nucleotide excision repair mechanism
Abstract
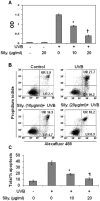
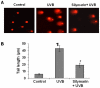
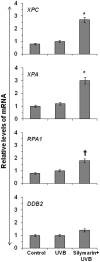
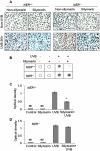
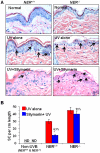
Solar ultraviolet (UV) radiation is a well recognized epidemiologic risk factor for melanoma and non-melanoma skin cancers. This observation has been linked to the accumulation of UVB radiation-induced DNA lesions in cells, and that finally lead to the development of skin cancers. Earlier, we have shown that topical treatment of skin with silymarin, a plant flavanoid from milk thistle (Silybum marianum), inhibits photocarcinogenesis in mice; however it is less understood whether chemopreventive effect of silymarin is mediated through the repair of DNA lesions in skin cells and that protect the cells from apoptosis. Here, we show that treatment of normal human epidermal keratinocytes (NHEK) with silymarin blocks UVB-induced apoptosis of NHEK in vitro. Silymarin reduces the amount of UVB radiation-induced DNA damage as demonstrated by reduced amounts of cyclobutane pyrimidine dimers (CPDs) and as measured by comet assay, and that ultimately may lead to reduced apoptosis of NHEK. The reduction of UV radiation-induced DNA damage by silymarin appears to be related with induction of nucleotide excision repair (NER) genes, because UV radiation-induced apoptosis was not blocked by silymarin in NER-deficient human fibroblasts. Cytostaining and dot-blot analysis revealed that silymarin repaired UV-induced CPDs in NER-proficient fibroblasts from a healthy individual but did not repair UV-induced CPD-positive cells in NER-deficient fibroblasts from patients suffering from xeroderma pigmentosum complementation-A disease. Similarly, immunohistochemical analysis revealed that silymarin did not reduce the number of UVB-induced sunburn/apoptotic cells in the skin of NER-deficient mice, but reduced the number of sunburn cells in their wild-type counterparts. Together, these results suggest that silymarin exert the capacity to reduce UV radiation-induced DNA damage and, thus, prevent the harmful effects of UV radiation on the genomic stability of epidermal cells.
Conflict of interest statement
Figures

Similar articles
-
Silibinin enhances the repair of ultraviolet B-induced DNA damage by activating p53-dependent nucleotide excision repair mechanism in human dermal fibroblasts.Oncotarget. 2015 Nov 24;6(37):39594-606. doi: 10.18632/oncotarget.5519. Oncotarget. 2015. PMID: 26447614 Free PMC article.
-
Apigenin prevents ultraviolet-B radiation induced cyclobutane pyrimidine dimers formation in human dermal fibroblasts.Mutat Res Genet Toxicol Environ Mutagen. 2017 Sep;821:28-35. doi: 10.1016/j.mrgentox.2017.06.002. Epub 2017 Jun 27. Mutat Res Genet Toxicol Environ Mutagen. 2017. PMID: 28735741
-
Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes.Cancer Prev Res (Phila). 2010 Feb;3(2):179-89. doi: 10.1158/1940-6207.CAPR-09-0044. Epub 2010 Jan 26. Cancer Prev Res (Phila). 2010. PMID: 20103727 Free PMC article.
-
Cell type and DNA damage specific response of human skin cells to environmental agents.Mutat Res. 2007 Jan 3;614(1-2):37-47. doi: 10.1016/j.mrfmmm.2006.06.009. Epub 2006 Aug 1. Mutat Res. 2007. PMID: 16879839 Review.
-
Solar UV damage to cellular DNA: from mechanisms to biological effects.Photochem Photobiol Sci. 2018 Dec 5;17(12):1842-1852. doi: 10.1039/c8pp00182k. Photochem Photobiol Sci. 2018. PMID: 30065996 Review.
Cited by
-
Bioactive grape proanthocyanidins enhance immune reactivity in UV-irradiated skin through functional activation of dendritic cells in mice.Cancer Prev Res (Phila). 2013 Mar;6(3):242-52. doi: 10.1158/1940-6207.CAPR-12-0320. Epub 2013 Jan 15. Cancer Prev Res (Phila). 2013. PMID: 23321928 Free PMC article.
-
Crosstalk Among UV-Induced Inflammatory Mediators, DNA Damage and Epigenetic Regulators Facilitates Suppression of the Immune System.Photochem Photobiol. 2017 Jul;93(4):930-936. doi: 10.1111/php.12687. Epub 2017 Feb 6. Photochem Photobiol. 2017. PMID: 27935057 Free PMC article. Review.
-
The Promising Role of Polyphenols in Skin Disorders.Molecules. 2024 Feb 15;29(4):865. doi: 10.3390/molecules29040865. Molecules. 2024. PMID: 38398617 Free PMC article. Review.
-
Chrysanthemum Morifolium Extract And Ascorbic Acid-2-Glucoside (AA2G) Blend Inhibits UVA-Induced Delayed Cyclobutane Pyrimidine Dimer (CPD) Production In Melanocytes.Clin Cosmet Investig Dermatol. 2019 Nov 13;12:823-832. doi: 10.2147/CCID.S223802. eCollection 2019. Clin Cosmet Investig Dermatol. 2019. PMID: 32009811 Free PMC article.
-
Silver nanoparticles protect human keratinocytes against UVB radiation-induced DNA damage and apoptosis: potential for prevention of skin carcinogenesis.Nanomedicine. 2015 Jul;11(5):1265-75. doi: 10.1016/j.nano.2015.02.024. Epub 2015 Mar 21. Nanomedicine. 2015. PMID: 25804413 Free PMC article.
References
-
- Housman TS, Feldman SR, Williford PM, Fleischer AB, Jr, Goldman ND, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48:425–429. - PubMed
-
- Murphy G, Young AR, Wulf HC, Kulms D, Schwarz T. The molecular determinants of sunburn cell formation. Exp Dermatol. 2001;10:155–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

