Short-term serum-free culture reveals that inhibition of Gsk3β induces the tumor-like growth of mouse embryonic stem cells
- PMID: 21731714
- PMCID: PMC3121758
- DOI: 10.1371/journal.pone.0021355
Short-term serum-free culture reveals that inhibition of Gsk3β induces the tumor-like growth of mouse embryonic stem cells
Abstract
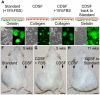
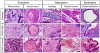
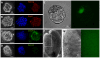
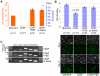
Here, we present evidence that the tumor-like growth of mouse embryonic stem cells (mESCs) is suppressed by short-term serum-free culture, which is reversed by pharmacological inhibition of Gsk3β. Mouse ESCs maintained under standard conditions using fetal bovine serum (FBS) were cultured in a uniquely formulated chemically-defined serum-free (CDSF) medium, namely ESF7, for three passages before being subcutaneously transplanted into immunocompromised mice. Surprisingly, the mESCs failed to produce teratomas for up to six months, whereas mESCs maintained under standard conditions generated well-developed teratomas in five weeks. Mouse ESCs cultured under CDSF conditions maintained the expression of Oct3/4, Nanog, Sox2 and SSEA1, and differentiated into germ cells in vivo. In addition, when mESCs were cultured under CDSF conditions supplemented with FBS, or when the cells were cultured under CDSF conditions followed by standard culture conditions, they consistently developed into teratomas. Thus, these results validate that the pluripotency of mESCs was not compromised by CDSF conditions. Mouse ESCs cultured under CDSF conditions proliferated significantly more slowly than mESCs cultured under standard conditions, and were reminiscent of Eras-null mESCs. In fact, their slower proliferation was accompanied by the downregulation of Eras and c-Myc, which regulate the tumor-like growth of mESCs. Remarkably, when mESCs were cultured under CDSF conditions supplemented with a pharmacological inhibitor of Gsk3β, they efficiently proliferated and developed into teratomas without upregulation of Eras and c-Myc, whereas mESCs cultured under standard conditions expressed Eras and c-Myc. Although the role of Gsk3β in the self-renewal of ESCs has been established, it is suggested with these data that Gsk3β governs the tumor-like growth of mESCs by means of a mechanism different from the one to support the pluripotency of ESCs.
Conflict of interest statement
Figures
Similar articles
-
Maintenance of murine embryonic stem cells' self-renewal and pluripotency with increase in proliferation rate by a bovine granulosa cell line-conditioned medium.Stem Cells Dev. 2011 Aug;20(8):1439-49. doi: 10.1089/scd.2010.0336. Epub 2011 Jan 12. Stem Cells Dev. 2011. PMID: 21126164
-
Inhibition of GSK3beta enhances both adhesive and signalling activities of beta-catenin in mouse embryonic stem cells.Biol Cell. 2010 Aug 27;102(10):549-60. doi: 10.1042/BC20100016. Biol Cell. 2010. PMID: 20626347
-
A modified EpiSC culture condition containing a GSK3 inhibitor can support germline-competent pluripotency in mice.PLoS One. 2014 Apr 15;9(4):e95329. doi: 10.1371/journal.pone.0095329. eCollection 2014. PLoS One. 2014. PMID: 24736627 Free PMC article.
-
Indole derivatives sustain embryonic stem cell self-renewal in long-term culture.Biosci Biotechnol Biochem. 2008 May;72(5):1242-8. doi: 10.1271/bbb.70717. Epub 2008 May 7. Biosci Biotechnol Biochem. 2008. PMID: 18460821
-
Defined and serum-free media support undifferentiated human embryonic stem cell growth.Stem Cells Dev. 2010 Jun;19(6):753-61. doi: 10.1089/scd.2009.0210. Stem Cells Dev. 2010. PMID: 19686051
Cited by
-
Cell culture of human gingival fibroblasts, oral cancer cells and mesothelioma cells with serum-free media, STK1 and STK2.Biomed Rep. 2014 Sep;2(5):644-648. doi: 10.3892/br.2014.306. Epub 2014 Jun 27. Biomed Rep. 2014. PMID: 25054004 Free PMC article.
-
Spatiotemporal clustering of the epigenome reveals rules of dynamic gene regulation.Genome Res. 2013 Feb;23(2):352-64. doi: 10.1101/gr.144949.112. Epub 2012 Oct 2. Genome Res. 2013. PMID: 23033340 Free PMC article.
-
Generation of organized germ layers from a single mouse embryonic stem cell.Nat Commun. 2014 May 30;5:4000. doi: 10.1038/ncomms5000. Nat Commun. 2014. PMID: 24873804 Free PMC article.
-
SMARCAD1 Contributes to the Regulation of Naive Pluripotency by Interacting with Histone Citrullination.Cell Rep. 2017 Mar 28;18(13):3117-3128. doi: 10.1016/j.celrep.2017.02.070. Cell Rep. 2017. PMID: 28355564 Free PMC article.
References
-
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. - PubMed
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
-
- Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. - PubMed
-
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science. 2007;318:1917–1920. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

