Cell cytoskeleton and tether extraction
- PMID: 21723813
- PMCID: PMC3127177
- DOI: 10.1016/j.bpj.2011.05.044
Cell cytoskeleton and tether extraction
Abstract
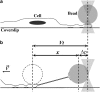
We perform a detailed investigation of the force × deformation curve in tether extraction from 3T3 cells by optical tweezers. Contrary to conventional wisdom about tethers extracted from cells, we find that actin filaments are present within them, so that a revised theory of tether pulling from cells is called for. We also measure steady and maximum tether force values significantly higher than previously published ones for 3T3 cells. Possible explanations for these differences are investigated. Further experimental support of the theory of force barriers for membrane tube extension is obtained. The potential of studies on tether pulling force × deformation for retrieving information on membrane-cytoskeleton interaction is emphasized.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Cell membrane biophysics with optical tweezers.Eur Biophys J. 2018 Jul;47(5):499-514. doi: 10.1007/s00249-017-1268-9. Epub 2017 Nov 21. Eur Biophys J. 2018. PMID: 29164289 Review.
-
Characteristics of a membrane reservoir buffering membrane tension.Biophys J. 1999 Oct;77(4):1992-2002. doi: 10.1016/S0006-3495(99)77040-2. Biophys J. 1999. PMID: 10512819 Free PMC article.
-
Substrate Stiffness Mediates Formation of Novel Cytoskeletal Structures in Fibroblasts during Cell-Microspheres Interaction.Int J Mol Sci. 2021 Jan 19;22(2):960. doi: 10.3390/ijms22020960. Int J Mol Sci. 2021. PMID: 33478069 Free PMC article.
-
Computational analysis of the tether-pulling experiment to probe plasma membrane-cytoskeleton interaction in cells.Phys Rev E Stat Nonlin Soft Matter Phys. 2009 Oct;80(4 Pt 1):041905. doi: 10.1103/PhysRevE.80.041905. Epub 2009 Oct 6. Phys Rev E Stat Nonlin Soft Matter Phys. 2009. PMID: 19905340 Free PMC article.
-
Force propagation across cells: mechanical coherence of dynamic cytoskeletons.Curr Opin Cell Biol. 2009 Feb;21(1):47-50. doi: 10.1016/j.ceb.2009.01.020. Epub 2009 Feb 7. Curr Opin Cell Biol. 2009. PMID: 19208463 Free PMC article. Review.
Cited by
-
Biomechanics of Neutrophil Tethers.Life (Basel). 2021 May 31;11(6):515. doi: 10.3390/life11060515. Life (Basel). 2021. PMID: 34073130 Free PMC article. Review.
-
Probing force in living cells with optical tweezers: from single-molecule mechanics to cell mechanotransduction.Biophys Rev. 2019 Oct;11(5):765-782. doi: 10.1007/s12551-019-00599-y. Epub 2019 Oct 14. Biophys Rev. 2019. PMID: 31612379 Free PMC article. Review.
-
Single-Cell Probe Force Studies to Identify Sox2 Overexpression-Promoted Cell Adhesion in MCF7 Breast Cancer Cells.Cells. 2020 Apr 10;9(4):935. doi: 10.3390/cells9040935. Cells. 2020. PMID: 32290242 Free PMC article.
-
Yielding elastic tethers stabilize robust cell adhesion.PLoS Comput Biol. 2014 Dec 4;10(12):e1003971. doi: 10.1371/journal.pcbi.1003971. eCollection 2014 Dec. PLoS Comput Biol. 2014. PMID: 25473833 Free PMC article.
-
Cell membrane biophysics with optical tweezers.Eur Biophys J. 2018 Jul;47(5):499-514. doi: 10.1007/s00249-017-1268-9. Epub 2017 Nov 21. Eur Biophys J. 2018. PMID: 29164289 Review.
References
-
- Lee C., Chen L.B. Dynamic behavior of endoplasmic reticulum in living cells. Cell. 1988;54:37–46. - PubMed
-
- Mattila P.K., Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008;9:446–454. - PubMed
-
- Davis D.M., Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat. Rev. Mol. Cell Biol. 2008;9:431–436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

