Nitric oxide metabolism in asthma pathophysiology
- PMID: 21718755
- PMCID: PMC3200501
- DOI: 10.1016/j.bbagen.2011.06.009
Nitric oxide metabolism in asthma pathophysiology
Abstract
Background: Asthma, a chronic inflammatory disease is typically characterized by bronchoconstriction and airway hyper-reactivity.
Scope of review: A wealth of studies applying chemistry, molecular and cell biology to animal model systems and human asthma over the last decade has revealed that asthma is associated with increased synthesis of the gaseous molecule nitric oxide (NO).
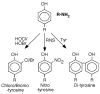
Major conclusion: The high NO levels in the oxidative environment of the asthmatic airway lead to greater formation of reactive nitrogen species (RNS) and subsequent oxidation and nitration of proteins, which adversely affect protein functions that are biologically relevant to chronic inflammation. In contrast to the high levels of NO and nitrated products, there are lower levels of beneficial S-nitrosothiols (RSNO), which mediate bronchodilation, due to greater enzymatic catabolism of RSNO in the asthmatic airways.
General significance: This review discusses the rapidly accruing data linking metabolic products of NO as critical determinants in the chronic inflammation and airway reactivity of asthma. This article is part of a Special Issue entitled Biochemistry of Asthma.
2011 Elsevier B.V. All rights reserved.
Figures
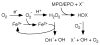
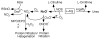
Similar articles
-
Oxidative and nitrosative events in asthma.Free Radic Biol Med. 2003 Aug 1;35(3):213-25. doi: 10.1016/s0891-5849(03)00278-8. Free Radic Biol Med. 2003. PMID: 12885584 Review.
-
Oxidative and nitrative stress in bronchial asthma.Antioxid Redox Signal. 2008 Apr;10(4):785-97. doi: 10.1089/ars.2007.1937. Antioxid Redox Signal. 2008. PMID: 18177234 Review.
-
Characterization of reactive nitrogen species in allergic asthma.Ann Allergy Asthma Immunol. 2014 Jan;112(1):18-22. doi: 10.1016/j.anai.2013.10.007. Epub 2013 Nov 6. Ann Allergy Asthma Immunol. 2014. PMID: 24331388 Review.
-
Nitric oxide and redox signaling in allergic airway inflammation.Antioxid Redox Signal. 2005 Jan-Feb;7(1-2):129-43. doi: 10.1089/ars.2005.7.129. Antioxid Redox Signal. 2005. PMID: 15650402 Review.
-
Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation.J Immunol. 2006 May 1;176(9):5587-97. doi: 10.4049/jimmunol.176.9.5587. J Immunol. 2006. PMID: 16622028
Cited by
-
Therapeutic Effect of Dipsacus asperoides C. Y. Cheng et T. M. Ai in Ovalbumin-Induced Murine Model of Asthma.Int J Mol Sci. 2019 Apr 15;20(8):1855. doi: 10.3390/ijms20081855. Int J Mol Sci. 2019. PMID: 30991656 Free PMC article.
-
Characterization and redox mechanism of asthma in the elderly.Oncotarget. 2016 May 3;7(18):25010-21. doi: 10.18632/oncotarget.7075. Oncotarget. 2016. PMID: 26843624 Free PMC article. Review.
-
Association between triglyceride-glucose index and fractional exhaled nitric oxide in adults with asthma from NHANES 2007-2012.Lipids Health Dis. 2024 Nov 3;23(1):335. doi: 10.1186/s12944-024-02323-6. Lipids Health Dis. 2024. PMID: 39488691 Free PMC article.
-
Associations between nitric oxide synthase genes and exhaled NO-related phenotypes according to asthma status.PLoS One. 2012;7(5):e36672. doi: 10.1371/journal.pone.0036672. Epub 2012 May 9. PLoS One. 2012. PMID: 22590587 Free PMC article.
-
Scrophularia koraiensis Nakai Attenuates Allergic Airway Inflammation via Suppression of NF-κB and Enhancement of Nrf2/HO-1 Signaling.Antioxidants (Basel). 2020 Jan 24;9(2):99. doi: 10.3390/antiox9020099. Antioxidants (Basel). 2020. PMID: 31991647 Free PMC article.
References
-
- Frieri M. Asthma concepts in the new millennium: update in asthma pathophysiology. Allergy Asthma Proc. 2005;26:83–88. - PubMed
-
- Iijima H, Duguet A, Eum SY, Hamid Q, Eidelman DH. Nitric oxide and protein nitration are eosinophil dependent in allergen-challenged mice. American journal of respiratory and critical care medicine. 2001;163:1233–1240. - PubMed
-
- Ricciardolo FL, Geppetti P, Mistretta A, Nadel JA, Sapienza MA, Bellofiore S, Di Maria GU. Randomised double-blind placebo-controlled study of the effect of inhibition of nitric oxide synthesis in bradykinin-induced asthma. Lancet. 1996;348:374–377. - PubMed
-
- Munzel T, Feil R, Mulsch A, Lohmann SM, Hofmann F, Walter U. Physiology and pathophysiology of vascular signaling controlled by guanosine 3′,5′-cyclic monophosphate-dependent protein kinase [corrected] Circulation. 2003;108:2172–2183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 RR024989/RR/NCRR NIH HHS/United States
- R01 HL069170-10/HL/NHLBI NIH HHS/United States
- P01 HL081064/HL/NHLBI NIH HHS/United States
- R01 HL069170/HL/NHLBI NIH HHS/United States
- P01 HL081064-05/HL/NHLBI NIH HHS/United States
- U10 HL109250/HL/NHLBI NIH HHS/United States
- UL1 RR024989-05/RR/NCRR NIH HHS/United States
- KL2 RR024990-05/RR/NCRR NIH HHS/United States
- RC1 HL099303/HL/NHLBI NIH HHS/United States
- P01 HL103453/HL/NHLBI NIH HHS/United States
- KL2 RR024990/RR/NCRR NIH HHS/United States
- R01 HL69170/HL/NHLBI NIH HHS/United States
- RC1 HL099303-02/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

