Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates?
- PMID: 21718746
- PMCID: PMC3159167
- DOI: 10.1016/j.vaccine.2011.06.083
Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates?
Abstract
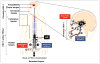
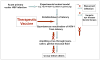
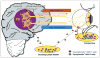
Herpes simplex virus type 1 and type 2 (HSV-1 and HSV-2)-specific CD8(+) T cells that reside in sensory ganglia, appear to control recurrent herpetic disease by aborting or reducing spontaneous and sporadic reactivations of latent virus. A reliable animal model is the ultimate key factor to test the efficacy of therapeutic vaccines that boost the level and the quality of sensory ganglia-resident CD8(+) T cells against spontaneous herpes reactivation from sensory neurons, yet its relevance has been often overlooked. Herpes vaccinologists are hesitant about using mouse as a model in pre-clinical development of therapeutic vaccines because they do not adequately mimic spontaneous viral shedding or recurrent symptomatic diseases, as occurs in human. Alternatives to mouse models are rabbits and guinea pigs in which reactivation arise spontaneously with clinical herpetic features relevant to human disease. However, while rabbits and guinea pigs develop spontaneous HSV reactivation and recurrent ocular and genital disease none of them can mount CD8(+) T cell responses specific to Human Leukocyte Antigen- (HLA-)restricted epitopes. In this review, we discuss the advantages and limitations of these animal models and describe a novel "humanized" HLA transgenic rabbit, which shows spontaneous HSV-1 reactivation, recurrent ocular disease and mounts CD8(+) T cell responses to HLA-restricted epitopes. Adequate investments are needed to develop reliable preclinical animal models, such as HLA class I and class II double transgenic rabbits and guinea pigs to balance the ethical and financial concerns associated with the rising number of unsuccessful clinical trials for therapeutic vaccine formulations tested in unreliable mouse models.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Therapeutic immunization with a mixture of herpes simplex virus 1 glycoprotein D-derived “asymptomatic” human CD8+ T-cell epitopes decreases spontaneous ocular shedding in latently infected HLA transgenic rabbits: association with low frequency of local PD-1+ TIM-3+ CD8+ exhausted T cells.J Virol. 2015 Jul;89(13):6619-32. doi: 10.1128/JVI.00788-15. J Virol. 2015. PMID: 25878105 Free PMC article.
-
Human Asymptomatic Epitope Peptide/CXCL10-Based Prime/Pull Vaccine Induces Herpes Simplex Virus-Specific Gamma Interferon-Positive CD107+ CD8+ T Cells That Infiltrate the Corneas and Trigeminal Ganglia of Humanized HLA Transgenic Rabbits and Protect against Ocular Herpes Challenge.J Virol. 2018 Jul 31;92(16):e00535-18. doi: 10.1128/JVI.00535-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29899087 Free PMC article.
-
A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes.J Immunol. 2010 Mar 1;184(5):2561-71. doi: 10.4049/jimmunol.0902322. Epub 2010 Feb 1. J Immunol. 2010. PMID: 20124097 Free PMC article.
-
Vaccines for Herpes Simplex: Recent Progress Driven by Viral and Adjuvant Immunology.Methods Mol Biol. 2020;2060:31-56. doi: 10.1007/978-1-4939-9814-2_2. Methods Mol Biol. 2020. PMID: 31617171 Review.
-
Use of the Guinea pig model of genital herpes to evaluate vaccines and antivirals: Review.Antiviral Res. 2020 Aug;180:104821. doi: 10.1016/j.antiviral.2020.104821. Epub 2020 Jun 13. Antiviral Res. 2020. PMID: 32544409 Free PMC article. Review.
Cited by
-
A Herpes Simplex Virus Type 1 Human Asymptomatic CD8+ T-Cell Epitopes-Based Vaccine Protects Against Ocular Herpes in a "Humanized" HLA Transgenic Rabbit Model.Invest Ophthalmol Vis Sci. 2015 Jun;56(6):4013-28. doi: 10.1167/iovs.15-17074. Invest Ophthalmol Vis Sci. 2015. PMID: 26098469 Free PMC article.
-
Intravaginal Zinc Oxide Tetrapod Nanoparticles as Novel Immunoprotective Agents against Genital Herpes.J Immunol. 2016 Jun 1;196(11):4566-75. doi: 10.4049/jimmunol.1502373. Epub 2016 Apr 27. J Immunol. 2016. PMID: 27183601 Free PMC article.
-
Evaluation of tissue interactions with mechanical elements of a transscleral drug delivery device.Pharmaceutics. 2012 Mar 12;4(1):212-29. doi: 10.3390/pharmaceutics4010212. Pharmaceutics. 2012. PMID: 24300189 Free PMC article.
-
Bolstering the Number and Function of HSV-1-Specific CD8+ Effector Memory T Cells and Tissue-Resident Memory T Cells in Latently Infected Trigeminal Ganglia Reduces Recurrent Ocular Herpes Infection and Disease.J Immunol. 2017 Jul 1;199(1):186-203. doi: 10.4049/jimmunol.1700145. Epub 2017 May 24. J Immunol. 2017. PMID: 28539429 Free PMC article.
-
Phenotypic and functional characterization of herpes simplex virus glycoprotein B epitope-specific effector and memory CD8+ T cells from symptomatic and asymptomatic individuals with ocular herpes.J Virol. 2015 Apr;89(7):3776-92. doi: 10.1128/JVI.03419-14. Epub 2015 Jan 21. J Virol. 2015. PMID: 25609800 Free PMC article.
References
-
- Morrison LA. Vaccines against genital herpes: progress and limitations. Drugs. 2002;62(8):1119–29. - PubMed
-
- Wilson KM, Triantafilou K, Morrison IE, Cherry RJ, Fernandez N. Single particle imaging of cell-surface HLA-DR tetramers. Biochem Soc Trans. 1997 May;25(2):360S. - PubMed
-
- Rubbo PA, Tuaillon E, Nagot N, Chentoufi AA, Bollore K, REYNES J, et al. HIV-1 infection impairs HSV-specific CD4+ and CD8+ T cell response by reducing Th1 cytokines and CCR5 ligand secretion. AIDS. 2011 In press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

