HTLV-1 bZIP factor enhances TGF-β signaling through p300 coactivator
- PMID: 21705495
- PMCID: PMC3158717
- DOI: 10.1182/blood-2010-12-326199
HTLV-1 bZIP factor enhances TGF-β signaling through p300 coactivator
Abstract
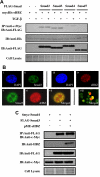
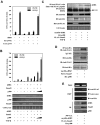
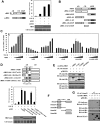
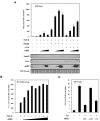
Human T-cell leukemia virus type 1 (HTLV-1) is an oncogenic retrovirus that is etiologically associated with adult T-cell leukemia. The HTLV-1 bZIP factor (HBZ), which is encoded by the minus strand of the provirus, is involved in both regulation of viral gene transcription and T-cell proliferation. We showed in this report that HBZ interacted with Smad2/3, and enhanced transforming growth factor-β (TGF-β)/Smad transcriptional responses in a p300-dependent manner. The N-terminal LXXLL motif of HBZ was responsible for HBZ-mediated TGF-β signaling activation. In a serial immunoprecipitation assay, HBZ, Smad3, and p300 formed a ternary complex, and the association between Smad3 and p300 was markedly enhanced in the presence of HBZ. In addition, HBZ could overcome the repression of the TGF-β response by Tax. Finally, HBZ expression resulted in enhanced transcription of Pdgfb, Sox4, Ctgf, Foxp3, Runx1, and Tsc22d1 genes and suppression of the Id2 gene; such effects were similar to those by TGF-β. In particular, HBZ induced Foxp3 expression in naive T cells through Smad3-dependent TGF-β signaling. Our results suggest that HBZ, by enhancing TGF-β signaling and Foxp3 expression, enables HTLV-1 to convert infected T cells into regulatory T cells, which is thought to be a critical strategy for virus persistence.
Figures


Similar articles
-
An interaction between the human T cell leukemia virus type 1 basic leucine zipper factor (HBZ) and the KIX domain of p300/CBP contributes to the down-regulation of tax-dependent viral transcription by HBZ.J Biol Chem. 2008 Aug 29;283(35):23903-13. doi: 10.1074/jbc.M803116200. Epub 2008 Jul 2. J Biol Chem. 2008. PMID: 18599479 Free PMC article.
-
EZH2 Activates HTLV-1 bZIP Factor-Mediated TGF-β Signaling in Adult T-Cell Leukemia.J Med Virol. 2024 Nov;96(11):e70025. doi: 10.1002/jmv.70025. J Med Virol. 2024. PMID: 39530290
-
Human T-cell leukemia virus type 1 (HTLV-1) bZIP factor requires cellular transcription factor JunD to upregulate HTLV-1 antisense transcription from the 3' long terminal repeat.J Virol. 2012 Sep;86(17):9070-8. doi: 10.1128/JVI.00661-12. Epub 2012 Jun 13. J Virol. 2012. PMID: 22696638 Free PMC article.
-
Adult T-cell leukemia-lymphoma as a viral disease: Subtypes based on viral aspects.Cancer Sci. 2021 May;112(5):1688-1694. doi: 10.1111/cas.14869. Epub 2021 Mar 30. Cancer Sci. 2021. PMID: 33630351 Free PMC article. Review.
-
[Adult T-cell leukemia induced by HTLV-1: before and after HBZ].Med Sci (Paris). 2010 Apr;26(4):391-6. doi: 10.1051/medsci/2010264391. Med Sci (Paris). 2010. PMID: 20412744 Review. French.
Cited by
-
The Road to HTLV-1-Induced Leukemia by Following the Subcellular Localization of HTLV-1-Encoded HBZ Protein.Front Immunol. 2022 Jun 23;13:940131. doi: 10.3389/fimmu.2022.940131. eCollection 2022. Front Immunol. 2022. PMID: 35812456 Free PMC article. Review.
-
HTLV-1 Alters T Cells for Viral Persistence and Transmission.Front Microbiol. 2018 Mar 20;9:461. doi: 10.3389/fmicb.2018.00461. eCollection 2018. Front Microbiol. 2018. PMID: 29615995 Free PMC article. Review.
-
The roles of acquired and innate immunity in human T-cell leukemia virus type 1-mediated diseases.Front Microbiol. 2012 Sep 3;3:323. doi: 10.3389/fmicb.2012.00323. eCollection 2012. Front Microbiol. 2012. PMID: 22969761 Free PMC article.
-
Molecular hallmarks of adult T cell leukemia.Front Microbiol. 2012 Sep 17;3:334. doi: 10.3389/fmicb.2012.00334. eCollection 2012. Front Microbiol. 2012. PMID: 23060864 Free PMC article.
-
Role of miRNAs in Human T Cell Leukemia Virus Type 1 Induced T Cell Leukemia: A Literature Review and Bioinformatics Approach.Int J Mol Sci. 2022 May 14;23(10):5486. doi: 10.3390/ijms23105486. Int J Mol Sci. 2022. PMID: 35628297 Free PMC article. Review.
References
-
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50(3):481–492. - PubMed
-
- Nicot C, Harrod RL, Ciminale V, Franchini G. Human T-cell leukemia/lymphoma virus type 1 nonstructural genes and their functions. Oncogene. 2005;24(39):6026–6034. - PubMed
-
- Grassmann R, Aboud M, Jeang KT. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene. 2005;24(39):5976–5985. - PubMed
-
- Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7(4):270–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

