T-bet controls severity of hypersensitivity pneumonitis
- PMID: 21699708
- PMCID: PMC3131238
- DOI: 10.1186/1476-9255-8-15
T-bet controls severity of hypersensitivity pneumonitis
Abstract
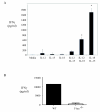
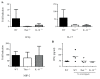
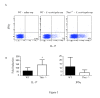
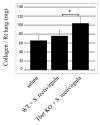
Hypersensitivity Pneumonitis (HP) is an interstitial lung disease that develops following repeated exposure to inhaled environmental antigens. The disease is characterized by alveolitis, granuloma formation and in some patients' fibrosis. IFNγ plays a critical role in HP; in the absence of IFNγ granuloma formation does not occur. However, recent studies using animal models of HP have suggested that HP is a Th17 disease calling into question the role of IFNγ. In this study, we report that initially IFNγ production is dependent on IL-18 and the transcription factor T-bet, however as the disease continues IFNγ production is IL-18-independent and partially T-bet dependent. Although IFNγ production is required for granuloma formation its role is distinct from that of T-bet. Mice that are deficient in T-bet and exposed to S. rectivirgula develop more severe disease characterized by an exacerbated Th17 cell response, decreased Th1 cell response, and increased collagen production in the lung. T-bet-mediated protection does not appear to be due to the development of a protective Th1 response; shifting the balance from a Th17 predominant response to a Th1 response by inhibition of IL-6 also results in lung pathology. The results from this study suggest that both Th1 and Th17 cells can be pathogenic in this model and that IFNγ and T-bet play divergent roles in the disease process.
Figures

Similar articles
-
Chronic hypersensitivity pneumonitis caused by Saccharopolyspora rectivirgula is not associated with a switch to a Th2 response.Am J Physiol Lung Cell Mol Physiol. 2016 Mar 1;310(5):L393-402. doi: 10.1152/ajplung.00305.2015. Epub 2015 Dec 30. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 26719148 Free PMC article.
-
Protein kinase D1 in myeloid lineage cells contributes to the accumulation of CXCR3+CCR6+ nonconventional Th1 cells in the lungs and potentiates hypersensitivity pneumonitis caused by S. rectivirgula.Front Immunol. 2024 Oct 11;15:1403155. doi: 10.3389/fimmu.2024.1403155. eCollection 2024. Front Immunol. 2024. PMID: 39464896 Free PMC article.
-
Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis.J Immunol. 2009 Jan 1;182(1):657-65. J Immunol. 2009. PMID: 19109199 Free PMC article.
-
Hypersensitivity pneumonitis and alpha-chemokines.Clin Ter. 2017 Mar-Apr;168(2):e140-e145. doi: 10.7417/CT.2017.1996. Clin Ter. 2017. PMID: 28383627 Review.
-
A cellular and molecular view of T helper 17 cell plasticity in autoimmunity.J Autoimmun. 2018 Feb;87:1-15. doi: 10.1016/j.jaut.2017.12.007. Epub 2017 Dec 22. J Autoimmun. 2018. PMID: 29275836 Review.
Cited by
-
TLR9-dependent IL-23/IL-17 is required for the generation of Stachybotrys chartarum-induced hypersensitivity pneumonitis.J Immunol. 2013 Jan 1;190(1):349-56. doi: 10.4049/jimmunol.1202225. Epub 2012 Nov 23. J Immunol. 2013. PMID: 23180821 Free PMC article.
-
Genetic variability of T cell responses in hypersensitivity pneumonitis identified using the BXD genetic reference panel.Am J Physiol Lung Cell Mol Physiol. 2020 Apr 1;318(4):L631-L643. doi: 10.1152/ajplung.00120.2019. Epub 2020 Jan 15. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 31940220 Free PMC article.
-
Chronic hypersensitivity pneumonitis caused by Saccharopolyspora rectivirgula is not associated with a switch to a Th2 response.Am J Physiol Lung Cell Mol Physiol. 2016 Mar 1;310(5):L393-402. doi: 10.1152/ajplung.00305.2015. Epub 2015 Dec 30. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 26719148 Free PMC article.
-
MAIT cells protect against pulmonary Legionella longbeachae infection.Nat Commun. 2018 Aug 22;9(1):3350. doi: 10.1038/s41467-018-05202-8. Nat Commun. 2018. PMID: 30135490 Free PMC article.
-
Novel acute hypersensitivity pneumonitis model induced by airway mycosis and high dose lipopolysaccharide.Respir Res. 2021 Oct 10;22(1):263. doi: 10.1186/s12931-021-01850-5. Respir Res. 2021. PMID: 34629055 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

